Prodrug Information
| Prodrug General Information | Top | |||||
|---|---|---|---|---|---|---|
| Prodrug ID |
D27YHV
|
|||||
| Prodrug Name |
Sulindac
|
|||||
| Synonyms |
Clinoril; 38194-50-2; Arthrocine; Sulindac sulfoxide; Sulindaco; Sulindacum; Aflodac; Mobilin; Sulreuma; MK 231; Sulindacum [INN-Latin]; Sulindaco [INN-Spanish]; MK-231; C20H17FO3S; CCRIS 3305; CHEBI:9352; BRN 2951842; Algocetil; Artribid; Citireuma; Clisundac; Imbaral; Reumofil; Sulinol; CHEMBL15770; Sudac; Arthrobid; Klinoril; CAS-38194-50-2; cis-Sulindac; Sulindac (Clinoril); Clinoril (TN); Sulindac [USAN:BAN:INN:JAN]; Moblilin; Sulindac (JP17/USP/INN)
Click to Show/Hide
|
|||||
| Indication | Osteoarthritis pain [ICD-11: MG30.31] | Approved outside US | [1] | |||
| Activation |
Prodrug 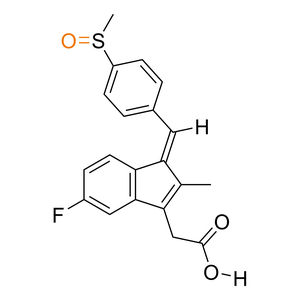
|

|
Parent Drug 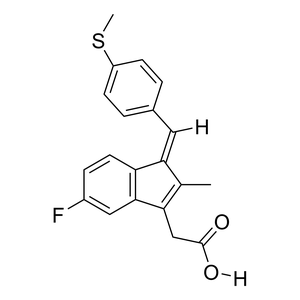
|
|||
| 2D MOL 3D MOL | 2D MOL 3D MOL | |||||
|
(1) Bioconversion Enzyme:
Methionine sulfoxide reductase
(EC 1.8)
|
[2] | |||||
| Prodrug Strategy |
Classical prodrug strategy
[Bioprecursor prodrug]
|
|||||
| Improved property |
Increase solubility
|
[4] | ||||
| Description |
Sulindac has a 100-fold increase in aqueous solubility then the parent drug.
|
[3] | ||||
| Formula |
C20H17FO3S
|
|||||
| Canonical SMILES |
CC\\1=C(C2=C(/C1=C\\C3=CC=C(C=C3)S(=O)C)C=CC(=C2)F)CC(=O)O
|
|||||
| InChI |
1S/C20H17FO3S/c1-12-17(9-13-3-6-15(7-4-13)25(2)24)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9-
|
|||||
| InChIKey |
MLKXDPUZXIRXEP-MFOYZWKCSA-N
|
|||||
| CAS Number |
CAS 38194-50-2
|
|||||
| PubChem Compound ID | ||||||
| ChEBI ID |
CHEBI:9352
|
|||||
| Parent Drug General Information | Top | |||||
|---|---|---|---|---|---|---|
| Parent Drug ID |
D5DGI3
|
|||||
| Parent Drug Name |
Sulindac sulfide
|
|||||
| Synonyms |
(Z)-Sulindac sulfide; UNII-6UVA8S2DEY; 32004-67-4; 6UVA8S2DEY; CHEMBL18797; MFCD00869764; Sulindac sulphide; CAS-49627-27-2; Sulindac sulfide, (Z)-; EINECS 256-403-9; UPCMLD-DP020; Sulindac Related Compound C; SCHEMBL2586650; DTXSID0049078; UPCMLD-DP020:001; CHEBI:95256; cid_5352624; (Z)-2-(5-fluoro-2-methyl-1-(4-(methylthio)benzylidene)-1H-inden-3-yl)acetic acid; HY-B1786; SFI; Tox21_113636; BDBM50110164; NSC747692; ZINC12404515; AKOS015965586; Tox21_113636_1; CCG-208108; NSC-747692; NCGC00161601-02; AC-20512; Sulindac Sulfide - CAS 32004-67-4; Sulindac sulfide, >=98% (HPLC), solid; UNM-0000306137; CS-0013817; 004S674
Click to Show/Hide
|
|||||
| Formula |
C20H17FO2S
|
|||||
| Canonical SMILES |
CC\\1=C(C2=C(/C1=C\\C3=CC=C(C=C3)SC)C=CC(=C2)F)CC(=O)O
|
|||||
| InChI |
1S/C20H17FO2S/c1-12-17(9-13-3-6-15(24-2)7-4-13)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9-
|
|||||
| InChIKey |
LFWHFZJPXXOYNR-MFOYZWKCSA-N
|
|||||
| CAS Number |
CAS 49627-27-2
|
|||||
| PubChem Compound ID | ||||||
| ChEBI ID |
CHEBI:75408
|
|||||
| Target and Pathway | Top | |||||
|---|---|---|---|---|---|---|
| Target(s) | Prostaglandin G/H synthase (COX) | Target Info | Inhibitor | [5] | ||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2010 | |||||
| REF 2 | Studies on the metabolism and biological activity of the epimers of sulindac. Drug Metab Dispos. 2011 Jun;39(6):1014-21. | |||||
| REF 3 | The disposition of sulindac. Clin Pharmacol Ther. 1977 Mar;21(3):326-35. | |||||
| REF 4 | Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008 Mar;7(3):255-70. | |||||
| REF 5 | Reduction of Sulindac to its active metabolite, sulindac sulfide: assay and role of the methionine sulfoxide reductase system. Biochem Biophys Res Commun. 2003 Dec 26;312(4):1005-10. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

