Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0T0LE
|
||||
| Former ID |
DIB001852
|
||||
| Drug Name |
IRX-4310
|
||||
| Synonyms |
AGN-194310; AGN-4310; ALRT-4310; LGD-4310; NRX-4310; RARa antagonist (oral, chemotherapy-induced neutropenia), Io Therapeutics; Retinoic acid receptor alpha antagonist (oral, chemotherapy-induced neutropenia), Io Therapeutics
|
||||
| Indication | Psoriasis [ICD9: 696; ICD10:L40] | Discontinued in Phase 3 | [546671] | ||
| Company |
Io Therapeutics Inc
|
||||
| Structure |
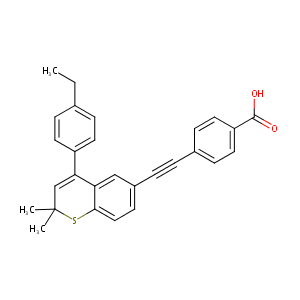
|
Download2D MOL |
|||
| Canonical SMILES |
C1(=CC(Sc2c1cc(C#Cc1ccc(C(=O)O)cc1)cc2)(C)C)c1ccc(cc1)C<br />C
|
||||
| CAS Number |
CAS 229961-45-9
|
||||
| Target and Pathway | |||||
| Target(s) | Retinoic acid receptor | Target Info | Antagonist | [527142] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

