Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02JOH
|
|||
| Former ID |
DNCL001969
|
|||
| Drug Name |
BMS-708163
|
|||
| Synonyms |
Avagacestat
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 2 | [1], [2] | |
| Company |
Bristol-Myers Squibb
|
|||
| Structure |
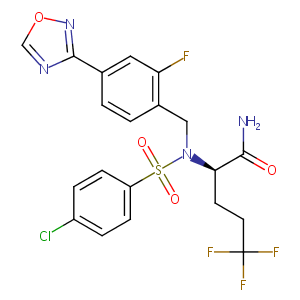 |
Download2D MOL |
||
| Formula |
C20H17ClF4N4O4S
|
|||
| Canonical SMILES |
C1=CC(=CC=C1S(=O)(=O)N(CC2=C(C=C(C=C2)C3=NOC=N3)F)C(CCC(F)(F)F)C(=O)N)Cl
|
|||
| InChI |
1S/C20H17ClF4N4O4S/c21-14-3-5-15(6-4-14)34(31,32)29(17(18(26)30)7-8-20(23,24)25)10-13-2-1-12(9-16(13)22)19-27-11-33-28-19/h1-6,9,11,17H,7-8,10H2,(H2,26,30)/t17-/m1/s1
|
|||
| InChIKey |
XEAOPVUAMONVLA-QGZVFWFLSA-N
|
|||
| CAS Number |
CAS 1146699-66-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
99437060, 103744604, 109693723, 120907854, 124757127, 125163931, 127340109, 131480772, 135263896, 135626624, 136340138, 136350035, 136367373, 136368035, 136946506, 137538919, 144115732, 152258831, 160647682, 162011642, 162037524, 162202740, 163415374, 163855192, 172918696, 174006713, 174526315, 178103103, 186007049, 188899567, 196409621, 198943217, 202544498, 223390085, 223704698, 224268119, 226649308, 242586972, 247490671
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6489). | |||
| REF 2 | ClinicalTrials.gov (NCT00890890) A Multicenter, Double Blind, Placebo-Controlled, Safety and Tolerability Study of BMS-708163 in Patients With Prodromal Alzheimer's Disease. U.S. National Institutes of Health. | |||
| REF 3 | Safety and tolerability of the gamma-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol. 2012 Nov;69(11):1430-40. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

