Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03LWG
|
|||
| Former ID |
DNCL002160
|
|||
| Drug Name |
Infigratinib
|
|||
| Synonyms |
BGJ398
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Cholangiocarcinoma [ICD-11: 2C12.10; ICD-10: C22.1] | Approved | [1] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 2 | [2], [3] | ||
| Company |
BridgeBio
|
|||
| Structure |
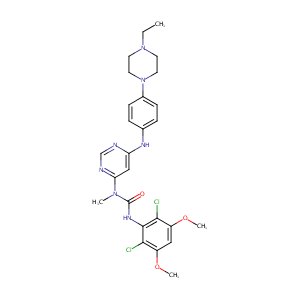 |
Download2D MOL |
||
| Formula |
C26H31Cl2N7O3
|
|||
| Canonical SMILES |
CCN1CCN(CC1)C2=CC=C(C=C2)NC3=CC(=NC=N3)N(C)C(=O)NC4=C(C(=CC(=C4Cl)OC)OC)Cl
|
|||
| InChI |
1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31)
|
|||
| InChIKey |
QADPYRIHXKWUSV-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 872511-34-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
124359248, 135610369, 136023525, 136348830, 136362079, 136946568, 137167188, 137602343, 144115702, 152159552, 152258531, 160647365, 162011595, 162205151, 163642798, 164034385, 164043528, 164193926, 172914340, 174006580, 174531137, 175426349, 177749165, 185975410, 189561513, 196409720, 198987912, 223366206, 223471433, 223705030, 224624345, 226706104, 242059884, 242459086, 248480244, 252160439, 252214977, 252449171, 252451765, 252543423, 252672020
|
|||
| ChEBI ID |
CHEBI:63451
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Fibroblast growth factor receptor (FGFR) | Target Info | Inhibitor | [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2021. Application Number: 214622. | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7877). | |||
| REF 3 | ClinicalTrials.gov (NCT02160041) BGJ398 for Patients With Tumors With FGFR Genetic Alterations. U.S. National Institutes of Health. | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

