Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05ROI
|
|||
| Drug Name |
Dabrafenib
|
|||
| Synonyms |
1195765-45-7; Dabrafenib (GSK2118436); Tafinlar; GSK2118436A; UNII-QGP4HA4G1B; GSK 2118436; QGP4HA4G1B; N-(3-(5-(2-aminopyrimidin-4-yl)-2-tert-butylthiazol-4-yl)-2-fluorophenyl)-2,6-difluorobenzenesulfonamide; CHEBI:75045; N-[3-[5-(2-Amino-4-pyrimidinyl)-2-(tert-butyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide; N-{3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide; GSK-2118436A; Dabrafenib [USAN:INN]; GSK2118436
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Melanoma [ICD-11: 2C30; ICD-9: 172] | Approved | [1] | |
| Structure |
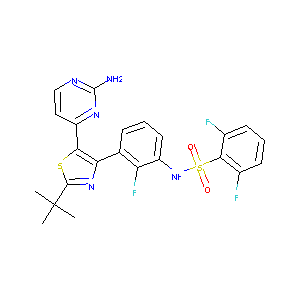 |
Download2D MOL |
||
| Formula |
C23H20F3N5O2S2
|
|||
| Canonical SMILES |
CC(C)(C)C1=NC(=C(S1)C2=NC(=NC=C2)N)C3=C(C(=CC=C3)NS(=O)(=O)C4=C(C=CC=C4F)F)F
|
|||
| InChI |
1S/C23H20F3N5O2S2/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25/h4-11,31H,1-3H3,(H2,27,28,29)
|
|||
| InChIKey |
BFSMGDJOXZAERB-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1195765-45-7
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:75045
|
|||
| ADReCS Drug ID | BADD_D00566 ; BADD_D00567 | |||
| Drug Resistance Mutation (DRM) | Top | |||
|---|---|---|---|---|
| DRM | DRM Info | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
| REF 2 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2011). | |||
| REF 3 | Caspase inhibitors: a review of recently patented compounds (2013-2015).Expert Opin Ther Pat. 2018 Jan;28(1):47-59. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

