Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0PM7Q
|
|||
| Former ID |
DCL000480
|
|||
| Drug Name |
AZD7762
|
|||
| Synonyms |
AZD7762; 860352-01-8; AZD-7762; (S)-5-(3-Fluorophenyl)-N-(piperidin-3-yl)-3-ureidothiophene-2-carboxamide; AZD 7762; UNII-5D822Y3L1H; 3-(Carbamoylamino)-5-(3-Fluorophenyl)-N-[(3s)-Piperidin-3-Yl]thiophene-2-Carboxamide; CHEMBL2041933; 5D822Y3L1H; J-502468; 5-(3-Fluorophenyl)-N-[(3s)-3-Piperidyl]-3-Ureido-Thiophene-2-Carboxamide; IAYGCINLNONXHY-LBPRGKRZSA-N; YDJ; AZD7762 hydrochloride; 5-(3-Fluorophenyl)-3-ureidothiophene-N-[(S)-piperidin-3-yl]-2-carboxamide; SCHEMBL1127614; GTPL7713; AOB1915; QCR-261; CHEBI:131156
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1 | [1], [2] | |
| Company |
AstraZeneca
|
|||
| Structure |
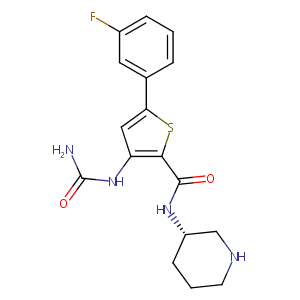 |
Download2D MOL |
||
| Formula |
C17H19FN4O2S
|
|||
| Canonical SMILES |
C1CC(CNC1)NC(=O)C2=C(C=C(S2)C3=CC(=CC=C3)F)NC(=O)N
|
|||
| InChI |
1S/C17H19FN4O2S/c18-11-4-1-3-10(7-11)14-8-13(22-17(19)24)15(25-14)16(23)21-12-5-2-6-20-9-12/h1,3-4,7-8,12,20H,2,5-6,9H2,(H,21,23)(H3,19,22,24)/t12-/m0/s1
|
|||
| InChIKey |
IAYGCINLNONXHY-LBPRGKRZSA-N
|
|||
| CAS Number |
CAS 860352-01-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
16231456, 23348024, 42207462, 75773863, 99437201, 117682512, 118847067, 124757322, 124950686, 125164126, 125329935, 131549273, 134213694, 135263746, 135727822, 136367458, 136368074, 136920283, 138468394, 144115659, 152258292, 152344041, 160647131, 160706048, 160868886, 162011707, 162037652, 163907978, 164045373, 170467355, 172912871, 174006971, 174561035, 186007043, 198971572, 203105583, 223366046, 224069960, 227368168, 244537251, 249582968, 252439982, 252450294
|
|||
| ChEBI ID |
CHEBI:131156
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7713). | |||
| REF 2 | ClinicalTrials.gov (NCT00473616) Phase I Single Ascending Dose/Multiple Ascending Dose in Patients Treated With AZD7762 and Irinotecan. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of AstraZeneca (2009). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

