Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0R3JB
|
|||
| Former ID |
DAP000057
|
|||
| Drug Name |
Mitoxantrone
|
|||
| Synonyms |
DHAD; DHAQ; Dihydroxyanthraquinone; MIX; Misostol; Mitoxanthrone; Mitoxantron; Mitoxantrona; Mitoxantronum; Mitozantrone; DHAQ HCl; Mitoxantrone [INN]; Mitozantrone hydrochloride; Mitoxantrone 2HCl; Liposome Encapsulated Mitoxantrone (LEM); Misostol (TN); Mitoxantrona [INN-Spanish]; Mitoxantrone (INN); Mitoxantrone (free base); Mitoxantronum [INN-Latin]; Novantrone (TN); AN-584/42007670; Novantrone(R) (mitoxantrone for injection concentrate); DHAQ (*Diacetate salt*); MITOXANTRONE, Mitoxantrone Hydrochloride, Mitoxantrone dihydrochloride, MITOXANTHRONE HYDROCHLORIDE; MITOXANTRONE, 1,4-DIHYDROXY-5,8-BIS({2-[(2-HYDROXYETHYL)AMINO]ETHYL}AMINO)ANTHRA-9,10-QUINONE; 1,4-Bis(2-(2-hydroxyethylamino)ethyl)amino)-5,8-dihydroxyanthraquinone; 1,4-DIHYDROXY-5,8-BIS({2-[(2-HYDROXYETHYL)AMINO]ETHYL}AMINO)-9,10-ANTHRACENEDIONE; 1,4-Dihydroxy-5,8-bis(2-((2-hydroxyethyl)amino)ethylamino)-9,10-anthracenedione; 1,4-Dihydroxy-5,8-bis(5-hydroxy-3-azapentylamino)anthrachinon; 1,4-Dihydroxy-5,8-bis[2-(2-hydroxyethylamino)ethylamino]anthracene-9,10-dione; 1,4-Dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]-9,10-anthracenedione; 1,4-dihydroxy-5,8-bis({2-[(2-hydroxyethyl)amino]ethyl}amino)anthra-9,10-quinone; 1,4-dihydroxy-5,8-bis({2-[(2-hydroxyethyl)amino]ethyl}amino)anthracene-9,10-dione; 5,8-Bis((2-((2-hydroxyethyl)amino)ethyl)amino)-1,4-dihydroxyanthraquinone; 9,10-Anthracenedione, 1,4-dihydroxy-5,8-bis((2-((2-hydroxyethyl)amino)ethyl)amino)-(9CI)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Approved | [1], [2] | |
| Non-hodgkin lymphoma [ICD-11: 2B33.5; ICD-10: C85.9] | Phase 1/2 | [3] | ||
| Therapeutic Class |
Analgesics
|
|||
| Company |
Serono
|
|||
| Structure |
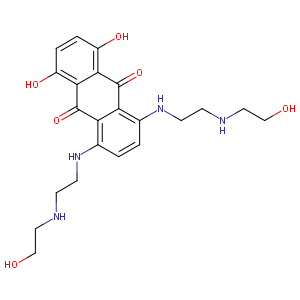 |
Download2D MOL |
||
| Formula |
C22H28N4O6
|
|||
| Canonical SMILES |
C1=CC(=C2C(=C1NCCNCCO)C(=O)C3=C(C=CC(=C3C2=O)O)O)NCCNCCO
|
|||
| InChI |
1S/C22H28N4O6/c27-11-9-23-5-7-25-13-1-2-14(26-8-6-24-10-12-28)18-17(13)21(31)19-15(29)3-4-16(30)20(19)22(18)32/h1-4,23-30H,5-12H2
|
|||
| InChIKey |
KKZJGLLVHKMTCM-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 65271-80-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
13376, 142727, 600874, 4873554, 7980008, 8152633, 11111482, 11111483, 11335691, 11360930, 11364549, 11367111, 11369673, 11372759, 11374669, 11377835, 11406669, 11406928, 11461902, 11466413, 11467533, 11484492, 11486226, 11488724, 11491405, 11492681, 11495469, 14710338, 14857298, 15047380, 17417295, 24769895, 29223317, 46394146, 46504608, 47216744, 47365147, 47440212, 47440213, 47515285, 47662243, 47736437, 48035072, 48035073, 48184960, 49698959, 49870599, 50111146, 53790842, 56352855
|
|||
| ChEBI ID |
CHEBI:50729
|
|||
| ADReCS Drug ID | BADD_D01480 ; BADD_D01481 | |||
| SuperDrug ATC ID |
L01DB07
|
|||
| SuperDrug CAS ID |
cas=065271809
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Enterobacterales | ||||
|
Studied Microbe: Escherichia coli Nissle 1917
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Metabolic Effect | Decrease activity | |||
| Description | Mitoxantrone can be metabolized by Escherichia coli Nissle 1917, which results in the decrease of the drug's activity. | |||
| Drug Resistance Mutation (DRM) | Top | |||
|---|---|---|---|---|
| DRM | DRM Info | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase II (TOP2) | Target Info | Modulator | [5] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7242). | |||
| REF 2 | Acute promyelocytic leukemic involvement of the optic nerves following mitoxantrone treatment for multiple sclerosis. J Neurol Sci. 2008 Oct 15;273(1-2):144-7. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep. 2015 Sep 29;5:14554. | |||
| REF 5 | Mitoxantrone, a topoisomerase II inhibitor, induces apoptosis of B-chronic lymphocytic leukaemia cells. Br J Haematol. 1998 Jan;100(1):142-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

