Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0S5LD
|
|||
| Former ID |
DNCL002440
|
|||
| Drug Name |
BAY 80-6946
|
|||
| Synonyms |
Copanlisib; BAY-80-6946; Aliqopa; UNII-WI6V529FZ9; BAY80-6946; WI6V529FZ9; AK172384; BAY 80-6946 (Copanlisib); Copanlisib [USAN:INN]; Copanlisib (USAN/INN); GTPL7875; SCHEMBL1655478; SCHEMBL13084037; Copanlisib (BAY 80-6946); DTXS
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Follicular lymphoma [ICD-11: 2A80] | Approved | [1] | |
| Non-hodgkin lymphoma [ICD-11: 2B33.5; ICD-10: C85.9] | Approved | [2] | ||
| Company |
Bayer HealthCare Pharmaceuticals
|
|||
| Structure |
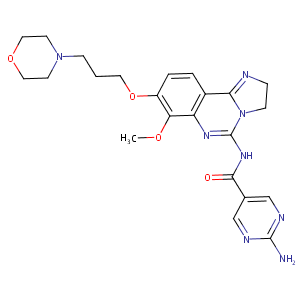 |
Download2D MOL |
||
| Formula |
C23H28N8O4
|
|||
| Canonical SMILES |
COC1=C(C=CC2=C3NCCN3C(=NC(=O)C4=CN=C(N=C4)N)N=C21)OCCCN5CCOCC5
|
|||
| InChI |
1S/C23H28N8O4/c1-33-19-17(35-10-2-6-30-8-11-34-12-9-30)4-3-16-18(19)28-23(31-7-5-25-20(16)31)29-21(32)15-13-26-22(24)27-14-15/h3-4,13-14,25H,2,5-12H2,1H3,(H2,24,26,27)
|
|||
| InChIKey |
MWYDSXOGIBMAET-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1032568-63-0
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110 and p110 activities in tumor cell lines and xenograft models.Mol Cancer Ther.2013 Nov;12(11):2319-30. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

