Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0T2QL
|
|||
| Former ID |
DIB019142
|
|||
| Drug Name |
cinnamic acid
|
|||
| Synonyms |
CINNAMIC ACID; TRANS-CINNAMIC ACID; 140-10-3; 621-82-9; (E)-Cinnamic acid; trans-3-Phenylacrylic acid; 3-Phenylacrylic acid; Phenylacrylic acid; Zimtsaeure; (2E)-3-phenylprop-2-enoic acid; 3-phenylprop-2-enoic acid; E-Cinnamic Acid; 3-Phenylpropenoic acid; (E)-3-phenylprop-2-enoic acid; trans-beta-Carboxystyrene; Benzenepropenoic acid; trans-Cinnamate; (E)-3-Phenyl-2-propenoic acid; (E)-cinnamate; Benzeneacrylic acid; trans-3-Phenyl-2-propenoic acid; Cinnamylic acid; Cinnamic acid, (E)-; t-Cinnamic acid; (2E)-3-Phenyl-2-propenoic
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
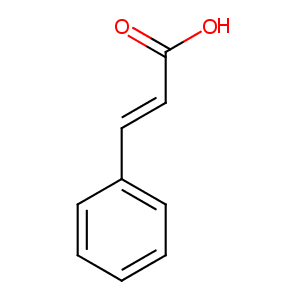 |
Download2D MOL
|
||
| Formula |
C9H8O2
|
|||
| Canonical SMILES |
C1=CC=C(C=C1)C=CC(=O)O
|
|||
| InChI |
1S/C9H8O2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7H,(H,10,11)/b7-6+
|
|||
| InChIKey |
WBYWAXJHAXSJNI-VOTSOKGWSA-N
|
|||
| CAS Number |
CAS 140-10-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
3713, 12623, 74712, 98281, 491236, 583919, 588530, 607059, 3135953, 7890710, 10299545, 10507636, 11533177, 14717783, 15219201, 22394956, 24848120, 24890291, 24893022, 24900955, 24900956, 25823625, 26703239, 29203962, 29217737, 36887530, 37608491, 46516668, 48421995, 49759521, 49856504, 49955076, 53788561, 57404626, 57924781, 80708217, 85164956, 85246717, 87565486, 87565727, 88529734, 92297555, 93166038, 99450362, 103131880, 103198631, 104632365, 104667294, 115001552, 117544895
|
|||
| ChEBI ID |
CHEBI:35697
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Nicotinic acid receptor (HCAR2) | Target Info | Agonist | [2] |
| Phosphomevalonate kinase (PMVK) | Target Info | Inhibitor | [3] | |
| KEGG Pathway | cAMP signaling pathway | |||
| Reactome | Class A/1 (Rhodopsin-like receptors) | |||
| G alpha (i) signalling events | ||||
| WikiPathways | GPCR ligand binding | |||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3203). | |||
| REF 2 | Phenolic acids suppress adipocyte lipolysis via activation of the nicotinic acid receptor GPR109A (HM74a/PUMA-G). J Lipid Res. 2009 May;50(5):908-14. | |||
| REF 3 | Inhibition of rat liver mevalonate pyrophosphate decarboxylase and mevalonate phosphate kinase by phenyl and phenolic compounds. Biochem J. 1979 Jul 1;181(1):143-51. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

