Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00NPP
|
|||
| Former ID |
DAP000715
|
|||
| Drug Name |
Zanamivir
|
|||
| Synonyms |
zanamivir; 139110-80-8; Relenza; 4-Guanidino-Neu5Ac2en; GANA; Zanamavir; MODIFIED SIALIC ACID; GG167; UNII-L6O3XI777I; GG 167; GR-121167X; Zanamivir (Relenza); Relenza (TN); 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid; GR 121167X; CHEMBL222813; L6O3XI777I; ZMR; CHEBI:50663; GANA (inhibitor); 5-Acetamido-2,6-anhydro-3,4,5-trideoxy-4-guanidino-D-glycero-D-galacto-non-2-enonic acid; GNA; AK163166; GG-167; DSSTox_CID_3749; DSSTox_RID_77184; DSSTox_GSID_23749; Zanamivir [USAN:INN:BAN]; HSDB 7437; C12H20N4O7; GANA; Zanamivir hydrate; GR121167X; Zanamivir (USAN/INN); (2R,3R,4S)-3-acetamido-4-(diaminomethylideneamino)-2-[(1R,2R)-1,2,3-trihydroxypropyl]-3,4-dihydro-2H-pyran-6-carboxylic acid; 4-Guanidino-NeueAc2en; 5-(acetylamino)-4-{[amino(imino)methyl]amino}-2,6-anhydro-3,4,5-trideoxy-D-glycero-D-galacto-non-2-enonic acid; ZNV; 4-guanidino-Neu5Ac2en; BAC-zanamivir
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Influenza virus infection [ICD-11: 1E30-1E32] | Approved | [1], [2] | |
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
GlaxoSmithKline
|
|||
| Structure |
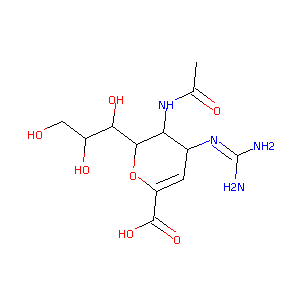 |
Download2D MOL |
||
| Formula |
C12H20N4O7
|
|||
| Canonical SMILES |
CC(=O)NC1C(C=C(OC1C(C(CO)O)O)C(=O)O)N=C(N)N
|
|||
| InChI |
1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1
|
|||
| InChIKey |
ARAIBEBZBOPLMB-UFGQHTETSA-N
|
|||
| CAS Number |
CAS 139110-80-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10295, 626318, 7847966, 7891225, 7980905, 8034575, 12014758, 14716772, 14801980, 14851079, 26709110, 26717829, 43118193, 46386819, 46391390, 46394328, 46508581, 48416708, 49738104, 53801134, 55161598, 57314153, 79665754, 81058936, 93166507, 104234237, 104321823, 118043618, 123109441, 126666923, 127446835, 134338003, 137003633, 138943627, 139483079, 144071866, 144203211, 144205743, 151980432, 152133958, 152258463, 160647298, 160963903, 162176829, 163981441, 164235531, 164831563, 170464662, 172089037, 175267628
|
|||
| ChEBI ID |
CHEBI:50663
|
|||
| ADReCS Drug ID | BADD_D02380 | |||
| SuperDrug ATC ID |
J05AH01
|
|||
| SuperDrug CAS ID |
cas=139110808
|
|||
| Drug Resistance Mutation (DRM) | Top | |||
|---|---|---|---|---|
| DRM | DRM Info | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Influenza Neuraminidase (Influ NA) | Target Info | Inhibitor | [3], [4] |
| KEGG Pathway | Other glycan degradation | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
| REF 3 | Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008 Apr;78(1):91-102. | |||
| REF 4 | Antiviral agents for influenza, hepatitis C and herpesvirus, enterovirus and rhinovirus infections. Med J Aust. 2001 Jul 16;175(2):112-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

