Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01ZUV
|
|||
| Former ID |
DNCL002334
|
|||
| Drug Name |
GDC-0425
|
|||
| Indication | Lymphoma [ICD-11: 2A80-2A86; ICD-9: 202.8, 208.9] | Phase 1 | [1] | |
| Company |
Genentech
|
|||
| Structure |
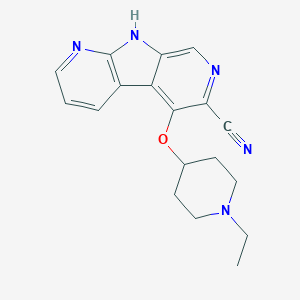 |
Download2D MOL |
||
| Formula |
C18H19N5O
|
|||
| Canonical SMILES |
CCN1CCC(CC1)OC2=C3C4=C(NC3=CN=C2C#N)N=CC=C4
|
|||
| InChI |
1S/C18H19N5O/c1-2-23-8-5-12(6-9-23)24-17-14(10-19)21-11-15-16(17)13-4-3-7-20-18(13)22-15/h3-4,7,11-12H,2,5-6,8-9H2,1H3,(H,20,22)
|
|||
| InChIKey |
XEZLBMHDUXSICI-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1627539-18-7
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01359696) A Study Evaluating the Safety, Tolerability, and Pharmacokinetics of GDC-0425 Administered With and Without Gemcitabine in Patients With Refractory Solid Tumors or Lymphoma. U.S. National Institutes of Health. | |||
| REF 2 | Quantitative assessment of BCL-2:BIM complexes as a pharmacodynamic marker for venetoclax (ABT-199). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

