Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02IWC
|
|||
| Former ID |
DCL000464
|
|||
| Drug Name |
AZD2624
|
|||
| Synonyms |
Pavinetant; AZD-2624; MLE4901; 941690-55-7; UNII-3U471ZVC5K; 3U471ZVC5K; AZ124752520; pavinetantum; Pavinetant [USAN]; SCHEMBL3587478; GTPL5775; CHEMBL3545233; CHEBI:140478; QYTBBBAHNIWFOD-NRFANRHFSA-N; BDBM50180193; AKOS032946112; DB11692; CS-7979; 4-Quinolinecarboxamide, 3-((methylsulfonyl)amino)-2-phenyl-N-((1S)-1-phenylpropyl)-; HY-14432; KB-74807; 3-methanesulfonamido-2-phenyl-N-[(1S)-1-phenylpropyl]quinoline-4-carboxamide; 3-[(methanesulfonyl)amino]-2-phenyl-N-[(1S)-1-phenylpropyl]quinoline-4-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | [; ] | Phase 2 | [1] | |
| Multiple sclerosis [ICD-11: 8A40; ICD-9: 340] | Phase 2 | [2] | ||
| Schizophrenia [ICD-11: 6A20] | Discontinued in Phase 2 | [3], [4] | ||
| Company |
AstraZeneca
|
|||
| Structure |
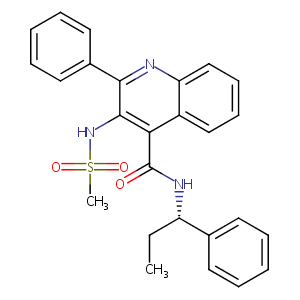 |
Download2D MOL
|
||
| Formula |
C26H25N3O3S
|
|||
| Canonical SMILES |
CCC(C1=CC=CC=C1)NC(=O)C2=C(C(=NC3=CC=CC=C32)C4=CC=CC=C4)NS(=O)(=O)C
|
|||
| InChI |
1S/C26H25N3O3S/c1-3-21(18-12-6-4-7-13-18)28-26(30)23-20-16-10-11-17-22(20)27-24(19-14-8-5-9-15-19)25(23)29-33(2,31)32/h4-17,21,29H,3H2,1-2H3,(H,28,30)/t21-/m0/s1
|
|||
| InChIKey |
QYTBBBAHNIWFOD-NRFANRHFSA-N
|
|||
| CAS Number |
CAS 941690-55-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:140478
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Neuromedin-K receptor (TACR3) | Target Info | Modulator | [5] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Reactome | G alpha (q) signalling events | |||
| WikiPathways | Gastrin-CREB signalling pathway via PKC and MAPK | |||
| Peptide GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00686998) A Phase IIa, Double-blind, Double-Dummy, Placebo-controlled, Randomized, Parallel-Group Study to Assess the Efficacy, Safety, Tolerability, and Pharmacokinetics of AZD2624 in Adult Schizophrenia Patients in AstraZeneca. | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800038259) | |||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5775). | |||
| REF 4 | ClinicalTrials.gov (NCT00686998) Phase IIA Study in Patients With Schizophrenia. U.S. National Institutes of Health. | |||
| REF 5 | The selective neurokinin 3 antagonist AZD2624 does not improve symptoms or cognition in schizophrenia: a proof-of-principle study.J Clin Psychopharmacol.2014 Apr;34(2):199-204. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

