Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02LPF
|
|||
| Former ID |
DAP000989
|
|||
| Drug Name |
Altretamine
|
|||
| Synonyms |
Altretamina; Altretaminum; HEXAMETHYLMELAMINE; HMM; HTM; HXM; Hemel; Hexalen; Hexastat; Hexinawas; Altretamine Bellon Brand; Altretamine Chiesi Brand; Altretamine Wassermann Brand; Bellon Brand of Altretamine; Chiesi Brand of Altretamine; MGI Pharma Brand of Altretamine; Rhone Poulenc Rorer Brand of Altretamine; Wassermann Brand of Altretamine; A 8723; ENT 50852; NC 195; Altretamina [INN-Spanish]; Altretaminum [INN-Latin]; Hexalen (TN); Hexalen, Altretamine; KB-913; Rhone-Poulenc Rorer Brand of Altretamine; Altretamine (USP/INN); Altretamine [USAN:INN:BAN]; No-s-triazine; N,N,N',N',N'',N''-hexamethyl-1,3,5-triazine-2,4,6-triamine; N~2~,N~2~,N~4~,N~4~,N~6~,N~6~-Hexamethyl-1,3,5-triazine-2,4,6-triamine; 2,4, 6-Tris(dimethylamino)-1,3,5-triazine; 2,4,6-Tris(dimethylami; 2,4,6-Tris(dimethylamino)-1,3,5-triazine; 2,4,6-Tris(dimethylamino-s-triazine; 2-N,2-N,4-N,4-N,6-N,6-N-hexamethyl-1,3,5-triazine-2,4,6-triamine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Ovarian cancer [ICD-11: 2C73; ICD-10: C56; ICD-9: 183] | Approved | [1], [2] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Structure |
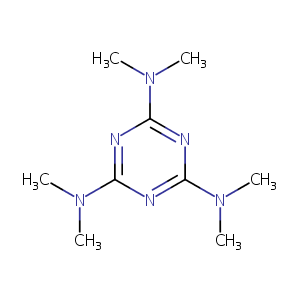 |
Download2D MOL |
||
| Formula |
C9H18N6
|
|||
| Canonical SMILES |
CN(C)C1=NC(=NC(=N1)N(C)C)N(C)C
|
|||
| InChI |
1S/C9H18N6/c1-13(2)7-10-8(14(3)4)12-9(11-7)15(5)6/h1-6H3
|
|||
| InChIKey |
UUVWYPNAQBNQJQ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 645-05-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
78208, 220082, 855523, 866238, 3297014, 7403876, 7861598, 7978484, 8149887, 8151440, 10531617, 11110789, 11110790, 11222335, 11335947, 11361186, 11364202, 11366764, 11369326, 11372141, 11374527, 11377488, 11405266, 11462158, 11466974, 11468094, 11484490, 11486807, 11488723, 11490766, 11492878, 11495122, 12013384, 14748896, 17396998, 17404646, 24278242, 24874728, 26612383, 26679553, 26746901, 26746902, 26746903, 29221302, 46505760, 47365225, 47440291, 47589031, 47589032, 47736514
|
|||
| ChEBI ID |
CHEBI:24564
|
|||
| ADReCS Drug ID | BADD_D00088 | |||
| SuperDrug ATC ID |
L01XX03
|
|||
| SuperDrug CAS ID |
cas=000645056
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Pseudomonadales | ||||
|
Studied Microbe: Pseudomonas putida
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Microbial Enzyme | N-demethylase | |||
| Metabolic Effect | Increase toxicity | |||
| Description | Altretamine can be metabolized by the N-demethylase of Pseudomonas putida, which results in the increase of the drug's toxicity. | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human Deoxyribonucleic acid (hDNA) | Target Info | Breaker | [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7112). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 019926. | |||
| REF 3 | The human gut chemical landscape predicts microbe-mediated biotransformation of foods and drugs. Elife. 2019 Jun 11;8:e42866. | |||
| REF 4 | Synergy of irofulven in combination with other DNA damaging agents: synergistic interaction with altretamine, alkylating, and platinum-derived agen... Cancer Chemother Pharmacol. 2008 Dec;63(1):19-26. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

