Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03WNG
|
|||
| Drug Name |
CORT-125134
|
|||
| Synonyms |
relacorilant; Relacorilant; UNII-2158753C7E; 2158753C7E; 1496510-51-0; Relacorilant [INN]; Relacorilant [WHO-DD]; Relacorilant [USAN:INN]; SCHEMBL15454999; WANIDIGFXJFFEL-SANMLTNESA-N; CORT125134; ZINC141949519; AKOS032945759; J3.651.009I; ((4aR)-1-(4-fluorophenyl)-6-(1-methyl-1H-pyrazole-4-sulfonyl)-1,4,5,6,7,8-hexahydro-4aH-pyrazolo(3,4-g)isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methanone; Methanone, ((4aR)-1-(4-fluorophenyl)-1,4,5,6,7,8-hexahydro-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4ah-pyrazolo(3,4-g)isoqui
Click to Show/Hide
|
|||
| Indication | Cushing disease [ICD-11: 5A70; ICD-10: E24; ICD-9: 255] | Phase 3 | [1] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1/2 | [2] | ||
| Alcohol dependence [ICD-11: 6C40.2; ICD-10: F10.2; ICD-9: 303] | Clinical trial | [3] | ||
| Company |
Corcept TherapeuticsMenlo Park, CA
|
|||
| Structure |
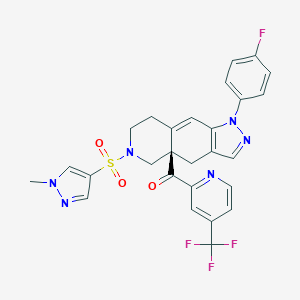 |
Download2D MOL |
||
| Formula |
C27H22F4N6O3S
|
|||
| Canonical SMILES |
CN1C=C(C=N1)S(=O)(=O)N2CCC3=CC4=C(CC3(C2)C(=O)C5=NC=CC(=C5)C(F)(F)F)C=NN4C6=CC=C(C=C6)F
|
|||
| InChI |
1S/C27H22F4N6O3S/c1-35-15-22(14-33-35)41(39,40)36-9-7-18-11-24-17(13-34-37(24)21-4-2-20(28)3-5-21)12-26(18,16-36)25(38)23-10-19(6-8-32-23)27(29,30)31/h2-6,8,10-11,13-15H,7,9,12,16H2,1H3/t26-/m0/s1
|
|||
| InChIKey |
WANIDIGFXJFFEL-SANMLTNESA-N
|
|||
| CAS Number |
CAS 1496510-51-0
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glucocorticoid receptor (NR3C1) | Target Info | Antagonist | [2], [3] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| NetPath Pathway | IL2 Signaling Pathway | |||
| TCR Signaling Pathway | ||||
| Pathway Interaction Database | Regulation of nuclear SMAD2/3 signaling | |||
| Signaling events mediated by HDAC Class II | ||||
| FOXA2 and FOXA3 transcription factor networks | ||||
| Glucocorticoid receptor regulatory network | ||||
| Regulation of Androgen receptor activity | ||||
| AP-1 transcription factor network | ||||
| Reactome | BMAL1:CLOCK,NPAS2 activates circadian gene expression | |||
| WikiPathways | Serotonin Receptor 4/6/7 and NR3C Signaling | |||
| SIDS Susceptibility Pathways | ||||
| Nuclear Receptors Meta-Pathway | ||||
| Endoderm Differentiation | ||||
| Hair Follicle Development: Cytodifferentiation (Part 3 of 3) | ||||
| Adipogenesis | ||||
| Circadian Clock | ||||
| Nuclear Receptors | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03697109) A Study of the Efficacy and Safety of Relacorilant in Patients With Endogenous Cushing Syndrome (GRACE). U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

