Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06DCL
|
|||
| Former ID |
DCL000157
|
|||
| Drug Name |
LY335979
|
|||
| Synonyms |
Zosuquidar HCl; Zosuquidar Trihydrochloride; LY 335979; LY-335979; Zosuquidar (TN); Zosuquidar trihydrochloride (USAN); RS-33295-198; Zosuquidar trihydrochloride, RS-33295-198, LY335979; (R)-4-((1aR,6R,10bS)-1,2-Difluoro-1,1a,6,10b-tetrahydrodibenzo(a,e)cyclopropa(c)cycloheptan-6-yl)-alpha-((5-quinoloyloxy)methyl)-1-piperazineethanol, trihydrochloride
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute myeloid leukaemia [ICD-11: 2A60] | Discontinued in Phase 3 | [1] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
Eli Lilly
|
|||
| Structure |
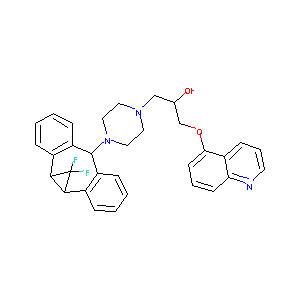 |
Download2D MOL |
||
| Formula |
C32H34Cl3F2N3O2
|
|||
| Canonical SMILES |
C1CN(CCN1CC(COC2=CC=CC3=C2C=CC=N3)O)C4C5=CC=CC=C5C6C(C6(F)F)C7=CC=CC=C47.Cl.Cl.Cl
|
|||
| InChI |
1S/C32H31F2N3O2.3ClH/c33-32(34)29-22-7-1-3-9-24(22)31(25-10-4-2-8-23(25)30(29)32)37-17-15-36(16-18-37)19-21(38)20-39-28-13-5-12-27-26(28)11-6-14-35-27;;;/h1-14,21,29-31,38H,15-20H2;3*1H/t21-,29-,30+,31?;;;/m1.../s1
|
|||
| InChIKey |
ZPFVQKPWGDRLHL-ZLYBXYBFSA-N
|
|||
| CAS Number |
CAS 167465-36-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
12014959, 14789967, 14936766, 46230217, 47208044, 50605721, 57347952, 99437179, 104437742, 125164087, 126671450, 131480893, 134338836, 135109630, 136340254, 136367440, 137249275, 152258808, 160647659, 160702421, 162011417, 162011631, 162037624, 162205163, 163907968, 170465595, 172080084, 174527858, 179322739, 184817594, 198952861, 223661255, 249814541, 251963144, 251971413, 252158852, 252814002
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Multidrug resistance protein 1 (ABCB1) | Target Info | Modulator | [2] |
| Multidrug resistance protein 3 (ABCB4) | Target Info | Modulator | [2] | |
| KEGG Pathway | ABC transporters | |||
| Bile secretion | ||||
| MicroRNAs in cancer | ||||
| NetPath Pathway | IL2 Signaling Pathway | |||
| TCR Signaling Pathway | ||||
| Pathway Interaction Database | HIF-1-alpha transcription factor network | |||
| Reactome | ABC-family proteins mediated transport | |||
| PPARA activates gene expression | ||||
| WikiPathways | Nuclear Receptors in Lipid Metabolism and Toxicity | |||
| Abacavir transport and metabolism | ||||
| Multi Drug Resistance Protein 1 (Glycoprotein 1) Regulation | ||||
| Integrated Pancreatic Cancer Pathway | ||||
| Allograft Rejection | ||||
| Drug Induction of Bile Acid Pathway | ||||
| Codeine and Morphine Metabolism | ||||
| Farnesoid X Receptor Pathway | ||||
| Regulation of Lipid Metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005159) | |||
| REF 2 | A Phase I trial of a potent P-glycoprotein inhibitor, zosuquidar trihydrochloride (LY335979), administered intravenously in combination with doxoru... Clin Cancer Res. 2004 May 15;10(10):3265-72. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

