Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06III
|
|||
| Former ID |
DIB020511
|
|||
| Drug Name |
NE10790
|
|||
| Synonyms |
NE 10790
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
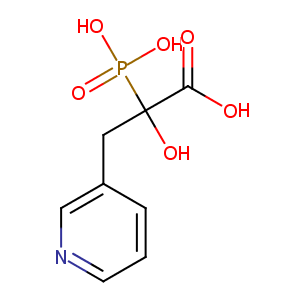 |
Download2D MOL
|
||
| Formula |
C8H10NO6P
|
|||
| Canonical SMILES |
C1=CC(=CN=C1)CC(C(=O)O)(O)P(=O)(O)O
|
|||
| InChI |
1S/C8H10NO6P/c10-7(11)8(12,16(13,14)15)4-6-2-1-3-9-5-6/h1-3,5,12H,4H2,(H,10,11)(H2,13,14,15)
|
|||
| InChIKey |
FJVYPXVLXQXDHM-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 152831-36-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Geranyltranstransferase (FDPS) | Target Info | Inhibitor | [2] |
| BioCyc | Superpathway of geranylgeranyldiphosphate biosynthesis I (via mevalonate) | |||
| Superpathway of cholesterol biosynthesis | ||||
| Trans, trans-farnesyl diphosphate biosynthesis | ||||
| Geranylgeranyldiphosphate biosynthesis | ||||
| KEGG Pathway | Terpenoid backbone biosynthesis | |||
| Metabolic pathways | ||||
| Biosynthesis of antibiotics | ||||
| NetPath Pathway | TCR Signaling Pathway | |||
| Panther Pathway | Cholesterol biosynthesis | |||
| Pathwhiz Pathway | Steroid Biosynthesis | |||
| Reactome | Cholesterol biosynthesis | |||
| Activation of gene expression by SREBF (SREBP) | ||||
| WikiPathways | Activation of Gene Expression by SREBP (SREBF) | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3165). | |||
| REF 2 | Structure-activity relationships among the nitrogen containing bisphosphonates in clinical use and other analogues: time-dependent inhibition of hu... J Med Chem. 2008 Apr 10;51(7):2187-95. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

