Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07IPB
|
|||
| Former ID |
DAP000650
|
|||
| Drug Name |
Valrubicin
|
|||
| Synonyms |
Valstar; Valrubicin [USAN]; Valstar Preservative Free; AD 32; Antibiotic AD 32; Valstar (TN); N-Trifluoroacetyladriamycin 14-valerate; N-Trifluoroacetyldoxorubicin 14-valerate; Trifluoroacetyladriamycin-14-valerate; Valrubicin (USP/INN); N-Trifluoroacetyladriamycin-14-valerate; Adriamycin, trifluoroacetyl-, 14-valerate; [2-oxo-2-[(2S,4S)-2,5,12-trihydroxy-4-[5-hydroxy-6-methyl-4-[(2,2,2-trifluoroacetyl)amino]oxan-2-yl]oxy-7-methoxy-6,11-dioxo-3,4-dihydro-1H-tetracen-2-yl]ethyl] pentanoate; (2S-cis)-2-(1,2,3,4,6,11-Hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((trifluoroacetyl)amino)-alpha-L-lyxo-hexopyranosyl)oxy)-2-naphthacenyl)-2-oxoethyl pentanoate; (2S-cis)-Pentanoic acid, 2-(1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((trifluoroacetyl)amino)-alpha-L-lyxo-hexopyranosyl)oxy)-2-naphth acenyl)-2-oxoethyl ester; (8S,10S)-8-Glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-((2,3,6-trideoxy-3-(2,2,2-trifluoroacetamido)-alpha-L-lyxo-hexopyranosyl)oxy)-5,12-naphthacenedione 8(sup 2)-valerate; Pentanoic acid, 2-((2S,4S)-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((trifluoroacetylamino)-, alpha-L-lysohexopyranoxyl)oxy)-2-naphthacenyl)-2-oxoethyl ester
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Bladder cancer [ICD-11: 2C94; ICD-9: 188] | Approved | [1] | |
| Psoriasis vulgaris [ICD-11: EA90; ICD-9: 696] | Phase 2 | [2] | ||
| Therapeutic Class |
Anticancer Agents
|
|||
| Structure |
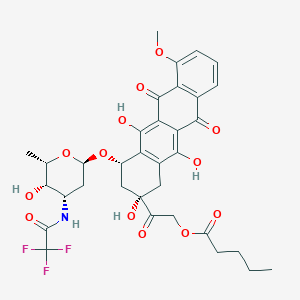 |
Download2D MOL
|
||
| Formula |
C34H36F3NO13
|
|||
| Canonical SMILES |
CCCCC(=O)OCC(=O)C1(CC(C2=C(C1)C(=C3C(=C2O)C(=O)C4=C(C3=O)C=CC=C4OC)O)OC5CC(C(C(O5)C)O)NC(=O)C(F)(F)F)O
|
|||
| InChI |
1S/C34H36F3NO13/c1-4-5-9-21(40)49-13-20(39)33(47)11-16-24(19(12-33)51-22-10-17(27(41)14(2)50-22)38-32(46)34(35,36)37)31(45)26-25(29(16)43)28(42)15-7-6-8-18(48-3)23(15)30(26)44/h6-8,14,17,19,22,27,41,43,45,47H,4-5,9-13H2,1-3H3,(H,38,46)/t14-,17-,19-,22-,27+,33-/m0/s1
|
|||
| InChIKey |
ZOCKGBMQLCSHFP-KQRAQHLDSA-N
|
|||
| CAS Number |
CAS 56124-62-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:135876
|
|||
| ADReCS Drug ID | BADD_D02330 | |||
| SuperDrug ATC ID |
L01DB09
|
|||
| SuperDrug CAS ID |
cas=056124620
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase II (TOP2) | Target Info | Inhibitor | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 3 | Metabolic activation of N-acylanthracyclines precedes their interaction with DNA topoisomerase II. NCI Monogr. 1987;(4):111-5. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

