Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07WXI
|
|||
| Former ID |
DNC000915
|
|||
| Drug Name |
Maribavir
|
|||
| Synonyms |
176161-24-3; Benzimidavir; (2S,3S,4R,5S)-2-(5,6-dichloro-2-(isopropylamino)-1H-benzo[d]imidazol-1-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol; 1263W94; UNII-PTB4X93HE1; PTB4X93HE1; 5,6-Dichloro-2-(isopropylamino)-1-(beta-L-ribofuranosyl)-1H-benzimidazole; 5,6-Dichloro-N-(1-methylethyl)-1-beta-L-ribofuranosyl-1H-benzimidazol-2-amine; (2S,3S,4R,5S)-2-[5,6-dichloro-2-(propan-2-ylamino)benzimidazol-1-yl]-5-(hydroxymethyl)oxolane-3,4-diol; Maribavir [USAN:INN:BAN]; Camvia; C15H19Cl2N3O4; Camvia(TM); Camvia (TN); Bzurea
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Cytomegalovirus infection [ICD-11: 1D82; ICD-9: 78.5] | Approved | [1] | |
| Company |
Takeda
|
|||
| Structure |
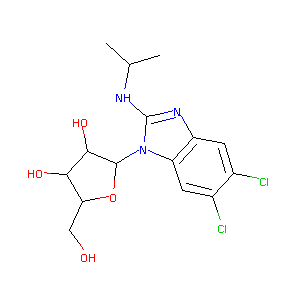 |
Download2D MOL |
||
| Formula |
C15H19Cl2N3O4
|
|||
| Canonical SMILES |
CC(C)NC1=NC2=CC(=C(C=C2N1C3C(C(C(O3)CO)O)O)Cl)Cl
|
|||
| InChI |
1S/C15H19Cl2N3O4/c1-6(2)18-15-19-9-3-7(16)8(17)4-10(9)20(15)14-13(23)12(22)11(5-21)24-14/h3-4,6,11-14,21-23H,5H2,1-2H3,(H,18,19)/t11-,12-,13-,14-/m0/s1
|
|||
| InChIKey |
KJFBVJALEQWJBS-XUXIUFHCSA-N
|
|||
| CAS Number |
CAS 176161-24-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
626300, 10306757, 12015036, 14804326, 14853350, 47206634, 50521885, 57405610, 71821537, 93302821, 103651231, 103842355, 109755304, 117368779, 118047322, 126629454, 128390115, 134339815, 134340205, 135078600, 137263772, 137267657, 142220709, 152101613, 160839470, 163099359, 163402541, 164824892, 184528158, 184546347, 198936541, 204433727, 210275491, 210281139, 223554910, 225069495, 227071991, 252345516, 252511550
|
|||
| SuperDrug ATC ID |
J05AX10
|
|||
| SuperDrug CAS ID |
cas=136470785
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Cytomegalovirus Ganciclovir kinase (CMV UL97) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 215596. | |||
| REF 2 | The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J Virol. 2003 Jan;77(2):905-14. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

