Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08SSP
|
|||
| Former ID |
DNCL002997
|
|||
| Drug Name |
Lersivirine
|
|||
| Synonyms |
cc-718; Lersivirine (UK-453061)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Human immunodeficiency virus infection [ICD-11: 1C62; ICD-9: 279.3] | Phase 2 | [1] | |
| Company |
ViiV Healthcare
|
|||
| Structure |
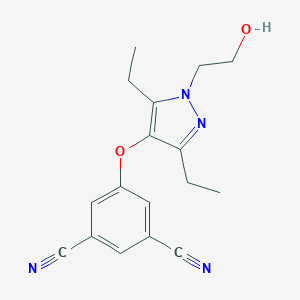 |
Download2D MOL |
||
| Formula |
C17H18N4O2
|
|||
| Canonical SMILES |
CCC1=C(C(=NN1CCO)CC)OC2=CC(=CC(=C2)C#N)C#N
|
|||
| InChI |
1S/C17H18N4O2/c1-3-15-17(16(4-2)21(20-15)5-6-22)23-14-8-12(10-18)7-13(9-14)11-19/h7-9,22H,3-6H2,1-2H3
|
|||
| InChIKey |
MCPUZZJBAHRIPO-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 473921-12-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human immunodeficiency virus Reverse transcriptase (HIV RT) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01254656) A Long Term Safety Study Of Lersivirine For The Treatment Of HIV-1 Infection In Subjects Who Have Completed Treatment With Lersivirine In Studies A5271015 And A5271022. U.S. National Institutes of Health. | |||
| REF 2 | Safety and tolerability of lersivirine, a nonnucleoside reverse transcriptase inhibitor, during a 28-day, randomized, placebo-controlled, Phase I clinical study in healthy male volunteers. Clin Ther.2010 Oct;32(11):1889-95. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

