Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09DJC
|
|||
| Former ID |
DNCL002746
|
|||
| Drug Name |
IRX4204
|
|||
| Synonyms |
220619-73-8; CHEMBL75133; UNII-877M97Z38Y; VTP-194204; 877M97Z38Y; KB-145960; SCHEMBL3437269; MolPort-042-665-869; ZINC1550770; IRX-4204; 3-Methyl-5-[2-methyl-2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-cyclopropyl]-penta-2,4-dienoic acid; BDBM50101445; DB11806; VTP 194204; (+)-VTP-194204; AGN 4204; (2E,4E)-3-Methyl-5-[(1S,2S)-2-methyl-2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-cyclopropyl]-penta-2,4-dienoic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Breast cancer [ICD-11: 2C60-2C65] | Phase 1 | [1] | |
| Non-small-cell lung cancer [ICD-11: 2C25.Y; ICD-9: 162] | Phase 1 | [1] | ||
| Pancreatic cancer [ICD-11: 2C10] | Phase 1 | [1] | ||
| Prostate cancer [ICD-11: 2C82.0; ICD-10: C61; ICD-9: 185] | Phase 1 | [2] | ||
| Company |
Io Therapeutics
|
|||
| Structure |
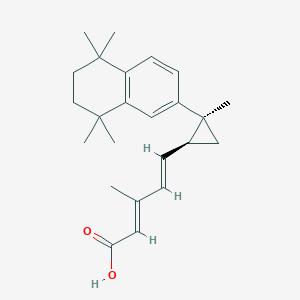 |
Download2D MOL |
||
| Formula |
C24H32O2
|
|||
| Canonical SMILES |
CC(=CC(=O)O)C=CC1CC1(C)C2=CC3=C(C=C2)C(CCC3(C)C)(C)C
|
|||
| InChI |
1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+/t18-,24-/m1/s1
|
|||
| InChIKey |
BOOOLEGQBVUTKC-NVQSDHBMSA-N
|
|||
| CAS Number |
CAS 220619-73-8
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Retinoic acid receptor RXR (RXR) | Target Info | Agonist | [1] |
| Retinoic acid receptor RXR-gamma (RXRG) | Target Info | Modulator | [3] | |
| KEGG Pathway | PPAR signaling pathway | |||
| Thyroid hormone signaling pathway | ||||
| Adipocytokine signaling pathway | ||||
| Pathways in cancer | ||||
| Transcriptional misregulation in cancer | ||||
| Thyroid cancer | ||||
| Small cell lung cancer | ||||
| Non-small cell lung cancer | ||||
| Pathway Interaction Database | Regulation of Androgen receptor activity | |||
| RXR and RAR heterodimerization with other nuclear receptor | ||||
| Retinoic acid receptors-mediated signaling | ||||
| a6b1 and a6b4 Integrin signaling | ||||
| Reactome | Nuclear Receptor transcription pathway | |||
| WikiPathways | Vitamin A and Carotenoid Metabolism | |||
| Adipogenesis | ||||
| Nuclear Receptors | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | ClinicalTrials.gov (NCT02438215) Study of IRX4204 for Treatment of Early Parkinson's Disease. U.S. National Institutes of Health. | |||
| REF 3 | Selective brain penetrable Nurr1 transactivator for treating Parkinson's disease.Oncotarget.2016 Feb 16;7(7):7469-79. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

