Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09URP
|
|||
| Former ID |
DIB009147
|
|||
| Drug Name |
DABIS MALEATE
|
|||
| Synonyms |
E-83-041; 1,4-Bis(2-chloroethyl)-1,4-diazoniabicyclo[2.2.1]heptane dimaleate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1 | [1] | |
| Structure |
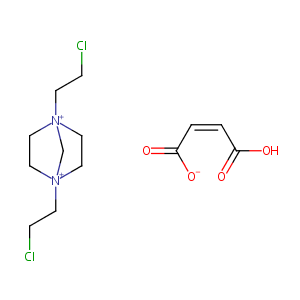 |
Download2D MOL |
||
| Formula |
C13H21Cl2N2O4+
|
|||
| Canonical SMILES |
C1C[N+]2(CC[N+]1(C2)CCCl)CCCl.C(=CC(=O)[O-])C(=O)O
|
|||
| InChI |
1S/C9H18Cl2N2.C4H4O4/c10-1-3-12-5-7-13(9-12,4-2-11)8-6-12;5-3(6)1-2-4(7)8/h1-9H2;1-2H,(H,5,6)(H,7,8)/q+2;/p-1/b;2-1-
|
|||
| InChIKey |
HKVBKOPISPWMSR-BTJKTKAUSA-M
|
|||
| CAS Number |
CAS 73387-70-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human Deoxyribonucleic acid (hDNA) | Target Info | Binder | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Phase I study of DABIS maleate given once every 3 weeks. Eur J Cancer. 1991;27(12):1635-7. | |||
| REF 2 | DNA damage and sequence specificity of DNA binding of the new anti-cancer agent 1,4-bis(2'-chloroethyl)-1,4-diazabicyclo-[2.2.1] heptane dimaleate (Dabis maleate)Broggini M1, Hartley JA, Mattes WB, Ponti M, Kohn KW, D'Incalci M.Author information1Laboratory of Cancer Chemotherapy, Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy.Erratum inBr J Cancer 1990 Jul;62(1):172. AbstractThe DNA damage and the sequence specificity of guanine-N7 alkylation produced by the novel, positively charged, antineoplastic agent 1,4-bis(2'-chloroethyl)-1,4-diazabicyclo-[2.2.1] heptane dimaleate (Dabis maleate) and its uncharged tertiary amine analogue 1,4-bis(2'-chloroethyl)-1,4-diazacyclohexane (Dabis analogue) were investigated in L1210 cells and isolated DNA. Both compounds are cytotoxic in vitro causing an arrest of L1210 cells in G2/M phase of the cell cycle. In isolated DNA, Dabis maleate alkylates guanine at the N7-position with some differences in specificity compared to other alkylating agents (e.g. nitrogen mustard). Significant differences are also evident between Dabis maleate and Dabis analogue, suggesting that Dabis analogue is not the sole alkylating species of Dabis maleate. Using the alkaline elution technique a moderate number of DNA interstrand cross-links were detected in L1210 cells treated with both compounds, which were completely repaired within 24 h. Dabis maleate and Dabis analogue do not cause DNA single strand breaks or DNA protein cross-links at the doses at which DNA interstrand cross-links were detected.PMID: 2393411 [PubMed - indexed for MEDLINE] PMCID: PMC1971404 Free PMC ArticleSharePublication Types, MeSH Terms, SubstancesPublication TypesResearch Support, Non-U.S. Gov'tMeSH TermsAnimalsAntineoplastic Agents/pharmacology*Base SequenceBicycloCompounds/pharmacology*Bicyclo Compounds, Heterocyclic*Bridged Compounds/pharmacology*Cell Survival/drug effectsDNA Damage*DNA, Neoplasm/drug effects*Dose-Response Relationship, DrugHumansInterphase/drug effectsLeukemia L1210/pathologyTumor Cells, Cultured/drug effectsSubstancesAntineoplastic AgentsBicyclo CompoundsBicyclo Compounds, HeterocyclicBridged CompoundsDNA, Neoplasm1,4-bis(2'-chloroethyl)-1,4-diazabicyclo(2.2.1)heptaneLinkOut - more resourcesFull Text SourcesEurope PubMed CentralPubMed CentralPubMed Central CanadaOther Literature SourcesCOS Scholar UniverseAccess more work from the authors - ResearchGateMiscellaneousNCI CPTAC Assay PortalPubMed Commons home Br J Cancer. 1990 Feb;61(2):285-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

