Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0CP4E
|
|||
| Former ID |
DAP000259
|
|||
| Drug Name |
Clonazepam
|
|||
| Synonyms |
Antelepsin; Antilepsin; Chlonazepam; Cloazepam; Clonazepamum; Clonex; Clonopin; Iktorivil; Kenoket; Klonopin; Landsen; Lktorivil; Lonazep; Melzap; Paxam; Ravotril; Rivatril; Rivoril; Rivotril; Solfidin; Klonopin Rapidly Disintegrating; DF2374250; RO4023; Ro 4023; Ro 54023; Alti-Clonazepam; Clonazepamum [INN-Latin]; Klonopin (TN); Ravotril (TN); Rivatril (TN); Rivotril (TN); Ro 4-8180; Ro 5-4023; Ro-5-4023; Clonazepam (JP15/USP/INN); Clonazepam [USAN:INN:BAN:JAN]; Ro 5-4023/B-7; 1,3-Dihydro-5-(o-chlorophenyl)-7-nitro-3H-1,4-benzodiazepin-2-one; 1,3-Dihydro-7-nitro-5-(2-chlorophenyl)-2H-1,4.benzodiazepin-2-one; 5-(2-Chloro-phenyl)-7-nitro-1,3-dihydro-benzo[e][1,4]diazepin-2-one; 5-(2-Chlorophenyl)-1,3-dihydro-7-nitro-2H-1,4-benzodiazepin-2-one; 5-(2-Chlorophenyl)-7-nitro-1,3-dihydro-2H-1,4-benzodiazepin-2-one; 5-(2-Chlorophenyl)-7-nitro-3H-1,4-benzodiazepin-2(1H)-one; 5-(2-chlorophenyl)-7-nitro-1,3-dihydro-1,4-benzodiazepin-2-one; 5-(o-Chlorophenyl)-1,3-dihydro-7-nitro-2H-1,4-benzodiazepin-2-one; 5-(o-Chlorophenyl)-7-nitro-1H-1,4-benzodiazepin-2(3H)-one; 5-(o-chlorophenyl)-7-nitro-1,3-dihydro-2H-1,4-benzodiazepin-2-one; 7-Nitro-5-(2-chlorophenyl)-3H-1,4-benzodiazepin-2(1H)-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Epilepsy [ICD-11: 8A60-8A68] | Approved | [1], [2] | |
| Seizure disorder [ICD-11: 8A6Z; ICD-9: 345.9, 780.3] | Phase 2 | [1], [3], [4] | ||
| Therapeutic Class |
Anticonvulsants
|
|||
| Company |
Roche Pharmaceuticals
|
|||
| Structure |
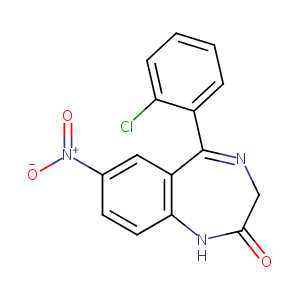 |
Download2D MOL |
||
| Formula |
C15H10ClN3O3
|
|||
| Canonical SMILES |
C1C(=O)NC2=C(C=C(C=C2)[N+](=O)[O-])C(=N1)C3=CC=CC=C3Cl
|
|||
| InChI |
1S/C15H10ClN3O3/c16-12-4-2-1-3-10(12)15-11-7-9(19(21)22)5-6-13(11)18-14(20)8-17-15/h1-7H,8H2,(H,18,20)
|
|||
| InChIKey |
DGBIGWXXNGSACT-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1622-61-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
447045, 629452, 4807399, 6242864, 7847346, 7978973, 8150128, 8151811, 10503355, 10509779, 10589180, 11109648, 11336148, 11361387, 11462359, 12146087, 14874486, 24892396, 29221957, 46507677, 47702094, 47736576, 48415805, 49884102, 49884205, 50876639, 56313737, 57321471, 85176029, 92307992, 92714482, 92729733, 93167162, 96026525, 96079552, 99302061, 103165964, 103930203, 104301655, 125536639, 126624103, 126655374, 126691438, 127729747, 131300953, 134337573, 134979678, 135315111, 136949632, 137002943
|
|||
| ChEBI ID |
CHEBI:3756
|
|||
| ADReCS Drug ID | BADD_D00501 | |||
| SuperDrug ATC ID |
N03AE01
|
|||
| SuperDrug CAS ID |
cas=001622613
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
| Studied Microbe: Gut microbiota unspecific | [5], [6], [7], [8] | |||
| Metabolic Reaction | Nitroreduction | |||
| Resulting Metabolite | 7-aminoclonazepam; 2-(2-amino-5-bromobenzoyl) pyridine | |||
| Metabolic Effect | Increase toxicity; Decrease activity | |||
| Description | Clonazepam can be metabolized to 7-aminoclonazepam and 2-(2-amino-5-bromobenzoyl) pyridine by gut microbiota through nitroreduction, which results in the increase of drug's toxicity and the decrease of the drug's activity. | |||
| Studied Microbe: Gut microbiota unspecific | [5], [6], [7], [9] | |||
| Microbial Enzyme | Nitroreductase | |||
| Metabolic Reaction | Nitroreduction | |||
| Resulting Metabolite | 7-aminoclonazepam | |||
| Metabolic Effect | Induce teratogenicity; Decrease activity | |||
| Description | Clonazepam can be metabolized to 7-aminoclonazepam by the nitroreductase of gut microbiota through nitroreduction, which results in the induction of the drug's teratogenicity and the decrease of the drug's activity. | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Gamma-aminobutyric acid receptor (GAR) | Target Info | Agonist | [10], [11] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6963). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 074569. | |||
| REF 3 | Characterization of GABAA receptor function in human temporal cortical neurons. J Neurophysiol. 1996 Apr;75(4):1458-71. | |||
| REF 4 | Residual effect of a 7-amino metabolite of clonazepam on GABAA receptor function in the nucleus reticularis thalami of the rat. Epilepsia. 2008 Oct;49(10):1803-8. | |||
| REF 5 | Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 2017 Jan;179:204-222. | |||
| REF 6 | Gut microbiota modulates drug pharmacokinetics. Drug Metab Rev. 2018 Aug;50(3):357-368. | |||
| REF 7 | Predicting and Understanding the Human Microbiome's Impact on Pharmacology. Trends Pharmacol Sci. 2019 Jul;40(7):495-505. | |||
| REF 8 | Personalized Mapping of Drug Metabolism by the Human Gut Microbiome. Cell. 2020 Jun 25;181(7):1661-1679.e22. | |||
| REF 9 | Cometabolism of microbes and host: implications for drug metabolism and drug-induced toxicity. Clin Pharmacol Ther. 2013 Nov;94(5):574-81. | |||
| REF 10 | Glutamate- and GABA-based CNS therapeutics. Curr Opin Pharmacol. 2006 Feb;6(1):7-17. | |||
| REF 11 | Hyperekplexia in neonates. Postgrad Med J. 2001 Sep;77(911):570-2. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

