Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0D3XM
|
|||
| Former ID |
DIB010466
|
|||
| Drug Name |
TAK-137
|
|||
| Indication | Psychiatric disorder [ICD-11: 6E8Z] | Phase 1 | [1] | |
| Structure |
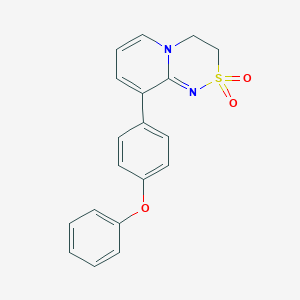 |
Download2D MOL |
||
| Formula |
C19H16N2O3S
|
|||
| Canonical SMILES |
C1CS(=O)(=O)N=C2N1C=CC=C2C3=CC=C(C=C3)OC4=CC=CC=C4
|
|||
| InChI |
1S/C19H16N2O3S/c22-25(23)14-13-21-12-4-7-18(19(21)20-25)15-8-10-17(11-9-15)24-16-5-2-1-3-6-16/h1-12H,13-14H2
|
|||
| InChIKey |
VKKLOYOLCCDGLD-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1358749-55-9
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glutamate receptor AMPA (GRIA) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02163915) Safety, Tolerability, and Pharmacokinetics of Multiple Rising Doses of TAK-137 in Adults With Attention-Deficit/Hyperactivity Disorder. U.S. National Institutes of Health. | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

