Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0D9HW
|
|||
| Former ID |
DAP000700
|
|||
| Drug Name |
Tenofovir
|
|||
| Synonyms |
Apropovir; PMPA; TFV; Tenefovir; GS 1275; GS 1278; GS1278; GNA & Tenofovir; HHA & Tenofovir; KS-5021; Viread (TN); Viread, Tenofovir; D,L-Tenofovir; PMPA-(R); Phosphonic acid, [[2-(6-amino-9H-purin-9; [(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethylphosphonic acid; Phosphonic acid, [[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]-(9CI); Phosphonic acid, [[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]-& Galanthus nivalis agglutinin (GNA); Phosphonic acid, [[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]-& Hippeastrum hybrid agglutinin(HHA); (R)-9-(2-Phosphonomethoxypropyl)adenine; (R)-9-(2-Phosphonylmethoxypropyl)adenine; (R)-PMPA
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Human immunodeficiency virus infection [ICD-11: 1C62; ICD-9: 279.3] | Approved | [1] | |
| Therapeutic Class |
Anti-HIV Agents
|
|||
| Company |
Gilead Sciences
|
|||
| Structure |
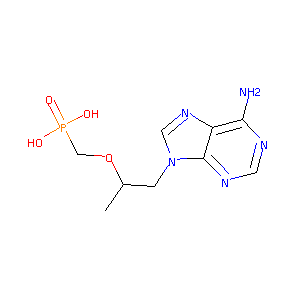 |
Download2D MOL |
||
| Formula |
C9H14N5O4P
|
|||
| Canonical SMILES |
CC(CN1C=NC2=C(N=CN=C21)N)OCP(=O)(O)O
|
|||
| InChI |
1S/C9H14N5O4P/c1-6(18-5-19(15,16)17)2-14-4-13-7-8(10)11-3-12-9(7)14/h3-4,6H,2,5H2,1H3,(H2,10,11,12)(H2,15,16,17)/t6-/m1/s1
|
|||
| InChIKey |
SGOIRFVFHAKUTI-ZCFIWIBFSA-N
|
|||
| CAS Number |
CAS 147127-20-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
610434, 3727050, 3727057, 7980754, 10304546, 12014981, 14873257, 46508131, 48424314, 50112788, 50437671, 57405565, 92719253, 99437132, 102979961, 103169192, 109738216, 121362228, 124757224, 125164028, 126610616, 126628295, 126655803, 127310209, 127310210, 127339001, 127339002, 129233080, 134337989, 135078595, 135606020, 135611180, 135682533, 135698226, 136367764, 136372374, 136905481, 137005935, 141613622, 151996120, 152034360, 152165365, 152246774, 152344570, 160963648, 162184452, 162793588, 163658367, 163813066, 171563543
|
|||
| ChEBI ID |
CHEBI:63625
|
|||
| ADReCS Drug ID | BADD_D02154 ; BADD_D02155 | |||
| SuperDrug ATC ID |
J05AF07
|
|||
| SuperDrug CAS ID |
cas=201341051
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bifidobacteriales | ||||
|
Studied Microbe: Gardnerella vaginalis
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Metabolic Effect | Decrease activity | |||
| Description | Tenofovir can be metabolized by Gardnerella vaginalis, which results in the decrease of the drug's activity. | |||
| Drug Resistance Mutation (DRM) | Top | |||
|---|---|---|---|---|
| DRM | DRM Info | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human immunodeficiency virus Reverse transcriptase (HIV RT) | Target Info | Inhibitor | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Anti-hepatitis B virus activity in vitro of combinations of tenofovir with nucleoside/nucleotide analogues. Antivir Chem Chemother. 2009;19(4):165-76. | |||
| REF 2 | Effects of Gut Microbiota on Drug Metabolism and Guidance for Rational Drug Use Under Hypoxic Conditions at High Altitudes. Curr Drug Metab. 2019;20(2):155-165. | |||
| REF 3 | Antiviral drugs in current clinical use. J Clin Virol. 2004 Jun;30(2):115-33. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

