Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0E3FD
|
|||
| Former ID |
DAP001035
|
|||
| Drug Name |
Bromazepam
|
|||
| Synonyms |
Bromazepamum; Calmepam; Compedium; Compendium; Creosedin; Durazanil; Lectopam; Lekotam; Lexaurin; Lexilium; Lexomil; Lexotan; Lexotanil; Normoc; Somalium; Ultramidol; Bromazepamum [Latin]; LA Xvii; KL 001; Ro 53350; Apo-Bromazepam; Brazepam (TN); Bromaze (TN); Bromazepam(USAN; Bromazepamum [INN-Latin]; Gen-Bromazepam; KL-001; Lectopam (TN); Lexotan (TN); Novo-bromazepam; Nu-Bromazepam; Ro 4-9253; Ro 5-3350; Bromazepam (JP15/USAN/INN); Bromazepam [USAN:INN:BAN:JAN]; 1,3-Dihydro-7-bromo-5-(2-pyridyl)-2H-1,4-benzodiazepin-2-one; 7-Bromo-1,3-dihydro-5-(2-pyridyl)-2H-1,4-benzdiazepin-2-one; 7-Bromo-1,3-dihydro-5-(2-pyridyl)-2H-1,4-benzodiazepin-2-one; 7-Bromo-5-(2-pyridyl)-3H-1,4-benzodiaxepin-2(1H)-one; 7-Bromo-5-(2-pyridyl)-3H-1,4-benzodiazepin-2(1H)-one; 7-bromo-5-pyridin-2-yl-1,3-dihydro-1,4-benzodiazepin-2-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Anxiety disorder [ICD-11: 6B00-6B0Z] | Approved | [1] | |
| Panic attacks [ICD-11: MB23.H] | Approved | [1] | ||
| Therapeutic Class |
Hypnotics and Sedatives
|
|||
| Company |
Hoffmann-La Roche pharmaceutical company
|
|||
| Structure |
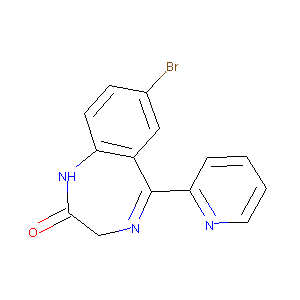 |
Download2D MOL |
||
| Formula |
C14H10BrN3O
|
|||
| Canonical SMILES |
C1C(=O)NC2=C(C=C(C=C2)Br)C(=N1)C3=CC=CC=N3
|
|||
| InChI |
1S/C14H10BrN3O/c15-9-4-5-11-10(7-9)14(17-8-13(19)18-11)12-3-1-2-6-16-12/h1-7H,8H2,(H,18,19)
|
|||
| InChIKey |
VMIYHDSEFNYJSL-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1812-30-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
426638, 5581001, 7848308, 7849654, 7978819, 8151628, 10511286, 14874497, 24891754, 29221605, 46505694, 49884085, 57321314, 57653933, 58106896, 75316922, 103179428, 104300596, 124360358, 126676319, 129495473, 134338299, 134979543, 136949630, 137001528, 141195787, 160964802, 163049283, 164837136, 164837137, 179148422, 184573827, 198951384, 198991799, 223454298, 223662261, 224436343, 226428244, 250119309
|
|||
| ChEBI ID |
CHEBI:31302
|
|||
| ADReCS Drug ID | BADD_D00299 | |||
| SuperDrug ATC ID |
N05BA08
|
|||
| SuperDrug CAS ID |
cas=001812302
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
| Studied Microbe: Gut microbiota unspecific | [2], [3], [4], [5] | |||
| Metabolic Reaction | Nitroreduction | |||
| Resulting Metabolite | 2-(2-amino-5-bromobenzoyl)pyridine | |||
| Metabolic Effect | Increase toxicity; Decrease activity | |||
| Description | Bromazepam can be metabolized to 2-(2-amino-5-bromobenzoyl)pyridine by gut microbiota through nitroreduction, which results in the increase of drug's toxicity and the decrease of the drug's activity. | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glutamate receptor AMPA (GRIA) | Target Info | Binder | [6] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drug information of Bromazepam, 2008. eduDrugs. | |||
| REF 2 | Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 2017 Jan;179:204-222. | |||
| REF 3 | Gut microbiota modulates drug pharmacokinetics. Drug Metab Rev. 2018 Aug;50(3):357-368. | |||
| REF 4 | Predicting and Understanding the Human Microbiome's Impact on Pharmacology. Trends Pharmacol Sci. 2019 Jul;40(7):495-505. | |||
| REF 5 | The metabolic effect of gut microbiota on drugs. Drug Metab Rev. 2020 Feb;52(1):139-156. | |||
| REF 6 | Glutamatergic and GABAergic modulations of ultrasonic vocalizations during maternal separation distress in mouse pups. Psychopharmacology (Berl). 2009 May;204(1):61-71. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

