Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0E6UZ
|
|||
| Former ID |
DNCL003478
|
|||
| Drug Name |
CUDC-907
|
|||
| Synonyms |
CUDC-907; 1339928-25-4; Fimepinostat; CUDC 907; UNII-3S9RX35S5X; CUDC907; 3S9RX35S5X; CHEMBL3622533; N-hydroxy-2-[[2-(6-methoxypyridin-3-yl)-4-morpholin-4-ylthieno[3,2-d]pyrimidin-6-yl]methyl-methylamino]pyrimidine-5-carboxamide; Fimepinostat [USAN]; PI3K/HDAC Inhibitor centn; MLS006010994; SCHEMBL1284705; GTPL8952; KS-00000TDO; EX-A742; AOB6775; DTXSID90712307; MolPort-023-293-550; JOWXJLIFIIOYMS-UHFFFAOYSA-N; HMS3656H04; BCP06870; s2759; BDBM50188961; 2341AH; ZINC73488511; ABP001045; AKOS026750340; SB16569; CUDC-907 (PI3K/HDAC Inhibi
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute myelogenous leukaemia [ICD-11: 2A41; ICD-10: C92.2] | Phase 1/2 | [1] | |
| Diffuse large B-cell lymphoma [ICD-11: 2A81; ICD-10: C83.3; ICD-9: 200] | Phase 1 | [2] | ||
| Neuroblastoma [ICD-11: 2D11.2] | Phase 1 | [3], [4] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1 | [3] | ||
| Company |
Curis
|
|||
| Structure |
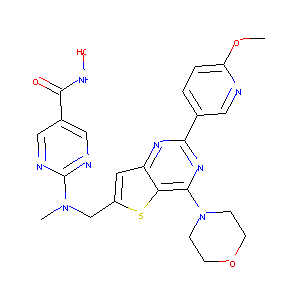 |
Download2D MOL |
||
| Formula |
C23H24N8O4S
|
|||
| Canonical SMILES |
CN(CC1=CC2=C(S1)C(=NC(=N2)C3=CN=C(C=C3)OC)N4CCOCC4)C5=NC=C(C=N5)C(=O)NO
|
|||
| InChI |
1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32)
|
|||
| InChIKey |
JOWXJLIFIIOYMS-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1339928-25-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04278768) Dose Escalation/ Expansion Trial of CA-4948 as Monotherapy and in Combination With Azacitidine or Venetoclax in Patients With AML or MDS. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of Curis. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 5 | Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling.Clin Cancer Res.2012 Aug 1;18(15):4104-13. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

