Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0G2MW
|
|||
| Former ID |
DAP000969
|
|||
| Drug Name |
Icosapent
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hyperglyceridemia [ICD-11: 5C80.1; ICD-10: R73.9; ICD-9: 790.29] | Approved | [1], [2] | |
| Thrombin deficiency [ICD-11: 3B14.Z] | Approved | [3], [4] | ||
| Hypertriglyceridemia [ICD-11: 5C80.1; ICD-10: E78.1, E78.3; ICD-9: 272.1, 427] | Phase 3 | [5] | ||
| Therapeutic Class |
Dietary supplement
|
|||
| Structure |
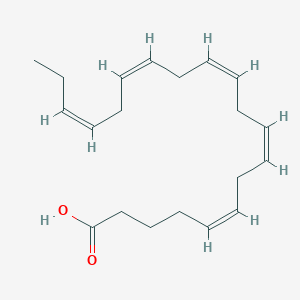 |
Download2D MOL |
||
| Formula |
C20H30O2
|
|||
| Canonical SMILES |
CCC=CCC=CCC=CCC=CCC=CCCCC(=O)O
|
|||
| InChI |
1S/C20H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h3-4,6-7,9-10,12-13,15-16H,2,5,8,11,14,17-19H2,1H3,(H,21,22)/b4-3-,7-6-,10-9-,13-12-,16-15-
|
|||
| InChIKey |
JAZBEHYOTPTENJ-JLNKQSITSA-N
|
|||
| CAS Number |
CAS 10417-94-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7848954, 14851023, 24132245, 44929533, 47205492, 49964825, 57373458, 71821310, 77834595, 87569697, 92308211, 99006112, 104179160, 117663994, 125307497, 125309298, 126666714, 135029488, 135114359, 135727811, 143077352, 144076075, 160686878, 162064799, 162262088, 175266496, 175268968, 175427134, 175611317, 178104013, 179117039, 223445352, 223680105, 224382965, 226494419, 242084018, 250134289, 252466658, 252816326
|
|||
| ChEBI ID |
CHEBI:28364
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Prostaglandin G/H synthase (COX) | Target Info | Binder | [6] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7441). | |||
| REF 2 | Drug information of Icosapent, Health Canada, 2007. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 4 | ClinicalTrials.gov (NCT00692718) N-3 Fatty Acids for the Prevention of Atrial Fibrillation in Patients With Acute Heart Failure. U.S. National Institutes of Health. | |||
| REF 5 | ClinicalTrials.gov (NCT01047683) Efficacy and Safety of AMR101 (Ethyl Icosapentate) in Patients With Fasting Triglyceride (Tg) Levels 500 and 2000 mg/dL. U.S. National Institutes of Health. | |||
| REF 6 | Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003 Mar;44(3):479-86. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

