Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0K2ZF
|
|||
| Former ID |
DNCL002815
|
|||
| Drug Name |
PH-797804
|
|||
| Synonyms |
PH-797804; 586379-66-0; PH797804; PH 797804; 3-(3-Bromo-4-((2,4-difluorobenzyl)oxy)-6-methyl-2-oxopyridin-1(2H)-yl)-N,4-dimethylbenzamide; UNII-SI09I1V827; UNII-GEL7GRJ3R6; GEL7GRJ3R6; CHEMBL1088751; CHEBI:82715; SI09I1V827; 3-{3-Bromo-4-[(2,4-Difluorobenzyl)oxy]-6-Methyl-2-Oxopyridin-1(2h)-Yl}-N,4-Dimethylbenzamide; PHA-797804; 1358027-80-1; 3-Bromo-4-((2,4-difluorobenzyl)oxy)-1-(5-((methylamino)carbonyl)-2-methylphenyl)-6-methylpyridin-2(1H)-one; 3hll; KCAJXIDMCNPGHZ-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chronic obstructive pulmonary disease [ICD-11: CA22; ICD-10: J44, J44.9] | Phase 2 | [1], [2] | |
| Company |
Pfizer
|
|||
| Structure |
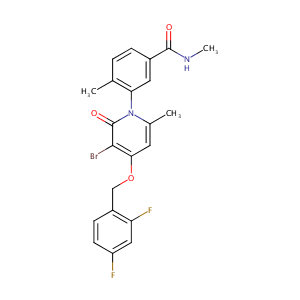 |
Download2D MOL |
||
| Formula |
C22H19BrF2N2O3
|
|||
| Canonical SMILES |
CC1=C(C=C(C=C1)C(=O)NC)N2C(=CC(=C(C2=O)Br)OCC3=C(C=C(C=C3)F)F)C
|
|||
| InChI |
1S/C22H19BrF2N2O3/c1-12-4-5-14(21(28)26-3)9-18(12)27-13(2)8-19(20(23)22(27)29)30-11-15-6-7-16(24)10-17(15)25/h4-10H,11H2,1-3H3,(H,26,28)
|
|||
| InChIKey |
KCAJXIDMCNPGHZ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 586379-66-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
37641639, 81087207, 99444412, 103744991, 103905624, 104148434, 135264394, 136340327, 136349570, 136367701, 136367826, 137276009, 139168666, 152258202, 160647038, 160838242, 160968999, 162011403, 162202643, 163098788, 163403917, 163846639, 164023443, 170482706, 180387142, 185992958, 196409740, 198942221, 204406251, 223366149, 223447436, 223573717, 223705084, 224194821, 228171297, 242059928, 249005282, 249860821, 250213402, 252070379, 252109954, 252160496, 252216057, 252438166
|
|||
| ChEBI ID |
CHEBI:82715
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7818). | |||
| REF 2 | ClinicalTrials.gov (NCT00559910) A Phase II, Study To Evaluate The Efficacy And Safety Of PH-797804 In Adults With Moderate To Severe Chronic Obstructive Pulmonary Disease (COPD).. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

