Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0L7UQ
|
|||
| Former ID |
DAP001142
|
|||
| Drug Name |
Prothionamide
|
|||
| Synonyms |
Ektebin; Peteha; Prothionamidum; Protionamid; Protionamida; Protionamide; Protionamidum; Protionizina; Tebeform; Trevintix; Tuberex; RP 9778; TH 1321; Prothionamide (JP15); Protionamida [INN-Spanish]; Protionamide (INN); Protionamide [INN:DCF]; Protionamidum [INN-Latin]; TH-1321; Trevintix (TN); 2-Propyl-4-pyridinecarbothioamide; 2-Propyl-4-thiocarbamoylpyridine; 2-Propyl-thioisonicotinamide; 2-Propylisonicotinylthioamide; 2-Propylthioisonicotinamide; 2-propylpyridine-4-carbothioamide; 4-Pyridinecarbothioamide, 2-propyl-(9CI); 9778 R.P; 9778 R.P.
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Tuberculosis [ICD-11: 1B10-1B1Z; ICD-10: A15-A19, B90] | Approved | [1] | |
| Therapeutic Class |
Antituberculosis Agents
|
|||
| Structure |
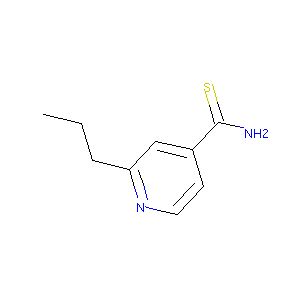 |
Download2D MOL |
||
| Formula |
C9H12N2S
|
|||
| Canonical SMILES |
CCCC1=NC=CC(=C1)C(=S)N
|
|||
| InChI |
1S/C9H12N2S/c1-2-3-8-6-7(9(10)12)4-5-11-8/h4-6H,2-3H2,1H3,(H2,10,12)
|
|||
| InChIKey |
VRDIULHPQTYCLN-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 14222-60-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
637882, 865168, 3189206, 4902981, 7848258, 9275772, 11373008, 11483793, 11487835, 11491779, 15120619, 25623620, 26612895, 26679417, 26749033, 26749034, 47589151, 49985848, 50085874, 50107508, 50600489, 57408475, 58003373, 71822746, 85174370, 87637593, 92124630, 92309187, 92712766, 109837069, 117656401, 121269830, 124407473, 124637868, 124766240, 124801318, 124974106, 125324968, 125340899, 126625256, 126656528, 126665572, 129515203, 131298799, 134222857, 134338387, 134988984, 135692316, 136992057, 137006684
|
|||
| ChEBI ID |
CHEBI:32066
|
|||
| SuperDrug ATC ID |
J04AD01
|
|||
| SuperDrug CAS ID |
cas=014222607
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Bacterial Fatty acid synthetase I (Bact inhA) | Target Info | Inhibitor | [1], [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Mechanism of thioamide drug action against tuberculosis and leprosy. J Exp Med. 2007 Jan 22;204(1):73-8. | |||
| REF 2 | Ability of the Ca2+ ionophores A23187 and ionomycin to mimic some of the effects of the tumor promoter 12-O-tetradecanoylphorbol-13-acetate on hydroperoxide production, ornithine decarboxylase activity, and DNA synthesis in mouse epidermis in vivo. Cancer Res. 1990 Sep 15;50(18):5806-12. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

