Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0L9UU
|
|||
| Former ID |
DAP000116
|
|||
| Drug Name |
Gentamicin
|
|||
| Synonyms |
Alcomicin; Apogen; Bristagen; Cidomycin; GENTAMYCIN; Garamycin; Garasol; Gentacidin; Gentacycol; Gentafair; Gentak; Gentamar; Gentamicina; Gentamicine; Gentamicins; Gentamicinum; Gentamycinum; Gentavet; Gentocin; Jenamicin; Refobacin; Uromycine; Garamycin Otic Solution; Genoptic Liquifilm; Gentamcin Sulfate; Gentamicin sulphate sterile; Refobacin TM; Gentamicin C1; G-Mycin; G-Myticin; Garamycin (TN); Gentamicin (BAN); Gentamicin (TN); Gentamicina [INN-Spanish]; Gentamicine [INN-French]; Gentamicinum [INN-Latin];Gentamycin-creme; Gentamycin-creme [German]; Ocu-Mycin; Spectro-Genta; U-Gencin; Genoptic S.O.P.; O-2-amino-2,3,4,6,7-pentadeoxy-6-(methylamino)-alpha-D-ribo-heptopyranosyl-(1-4)-O-(3-deoxy-4-C-methyl-3-(methylamino)-beta-L-arabinopyranosyl-(1-6))-2-deoxy-D-streptamine; (1R,2S,3S,4R,6S)-4,6-diamino-3-[3-deoxy-4-C-methyl-3-(methylamino)-beta-L-arabinopyranosyloxy]-2-hydroxycyclohexyl 2-amino-2,3,4,6,7-pentadeoxy-6-(methylamino)-beta-L-lyxo-heptopyranoside; (1R,2S,3S,4R,6S)-4,6-diamino-3-{[3-deoxy-4-C-methyl-3-(methylamino)-beta-L-arabinopyranosyl]oxy}-2-hydroxycyclohexyl (6x)-2-amino-2,3,4,6,7-pentadeoxy-6-(methylamino)-alpha-D-erythro-heptopyranoside; (2R,3R,4R,5R)-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-[1-(methylamino)ethyl]oxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol; 2-[4,6-diamino-3-[3-amino-6-[1-(methylamino)ethyl]oxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol; 4,6-diamino-3-{[3-deoxy-4-c-methyl-3-(methylamino)pentopyranosyl]oxy}-2-hydroxycyclohexyl 2-amino-2,3,4,6,7-pentadeoxy-6-(methylamino)heptopyranoside
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Bacterial infection [ICD-11: 1A00-1C4Z; ICD-10: A00-B99] | Approved | [1], [2] | |
| Therapeutic Class |
Antibiotics
|
|||
| Company |
Sandoz
|
|||
| Structure |
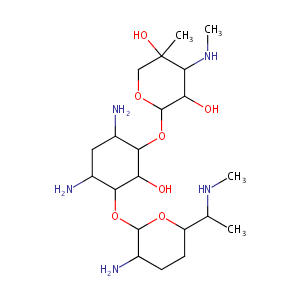 |
Download2D MOL |
||
| Formula |
C21H43N5O7
|
|||
| Canonical SMILES |
CC(C1CCC(C(O1)OC2C(CC(C(C2O)OC3C(C(C(CO3)(C)O)NC)O)N)N)N)NC
|
|||
| InChI |
1S/C21H43N5O7/c1-9(25-3)13-6-5-10(22)19(31-13)32-16-11(23)7-12(24)17(14(16)27)33-20-15(28)18(26-4)21(2,29)8-30-20/h9-20,25-29H,5-8,22-24H2,1-4H3
|
|||
| InChIKey |
CEAZRRDELHUEMR-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1403-66-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
4652583, 8152203, 29222600, 46506523, 49972189, 53787495, 57321817, 74460187, 92712501, 96024706, 103135455, 104303599, 117535962, 124766025, 126407615, 126625531, 126661266, 126691181, 127340104, 127340105, 127340106, 134337440, 134980500, 135650301, 137239444, 142147875, 152100630, 160964140, 196105377, 223658860, 226847630, 242084438, 246167234, 250134950
|
|||
| ADReCS Drug ID | BADD_D01016 ; BADD_D01017 | |||
| SuperDrug ATC ID |
D06AX07; J01GB03; S01AA11; S02AA14; S03AA06
|
|||
| SuperDrug CAS ID |
cas=001403663
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Bacterial 30S ribosomal RNA (Bact 30S rRNA) | Target Info | Modulator | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2427). | |||
| REF 2 | How many modes of action should an antibiotic have Curr Opin Pharmacol. 2008 Oct;8(5):564-73. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

