Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0ML2V
|
|||
| Former ID |
DIB004814
|
|||
| Drug Name |
RALURIDINE
|
|||
| Synonyms |
FCU; FddClUrd; BW-935U83; Raluridine; 5-Chloro-2',3'-dideoxy-3'-fluorouridine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Human immunodeficiency virus infection [ICD-11: 1C62; ICD-9: 279.3] | Phase 2 | [1], [2] | |
| Structure |
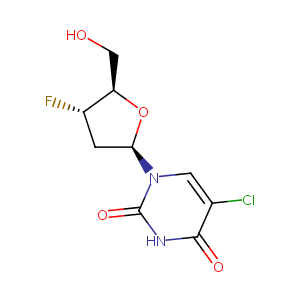 |
Download2D MOL |
||
| Formula |
C9H10ClFN2O4
|
|||
| Canonical SMILES |
C1C(C(OC1N2C=C(C(=O)NC2=O)Cl)CO)F
|
|||
| InChI |
1S/C9H10ClFN2O4/c10-4-2-13(9(16)12-8(4)15)7-1-5(11)6(3-14)17-7/h2,5-7,14H,1,3H2,(H,12,15,16)/t5-,6+,7+/m0/s1
|
|||
| InChIKey |
WKVDSZYIGHLONN-RRKCRQDMSA-N
|
|||
| CAS Number |
CAS 119644-22-3
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human immunodeficiency virus Reverse transcriptase (HIV RT) | Target Info | Inhibitor | [3], [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00002338) The Safety and Effectiveness of 935U83 in HIV-Infected Patients. U.S. National Institutes of Health. | |||
| REF 2 | Safety and pharmacokinetics of 5-chloro-2',3'-dideoxy-3'-fluorouridine (935U83) following oral administration of escalating single doses in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 1996 Dec;40(12):2842-7. | |||
| REF 3 | 5-Chloro-2',3'-dideoxy-3'-fluorouridine (935U83), a selective anti-human immunodeficiency virus agent with an improved metabolic and toxicological profile. Antimicrob Agents Chemother. 1994 Jul;38(7):1590-603. | |||
| REF 4 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

