Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0OR8W
|
|||
| Former ID |
DIB012163
|
|||
| Drug Name |
ETIPREDNOL DICLOACETATE
|
|||
| Synonyms |
BNP-166; Cronaze (Ivax); Ethinase (Ivax); Etiprednol dicloacetate < USAN; Respicort (Ivax); (11beta,17alpha)-17-(2,2-Dichloroacetoxy)-11-hydroxy-3-oxoandrosta-1,4-diene-17-carboxylic acid ethyl ester
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Rhinitis [ICD-11: FA20; ICD-10: J31.0] | Phase 2 | [1] | |
| Company |
Teva Pharmaceutical USA
|
|||
| Structure |
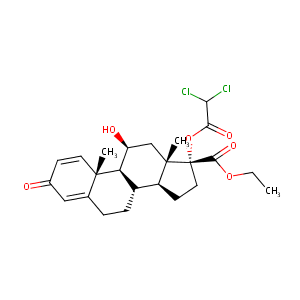 |
Download2D MOL |
||
| Formula |
C24H30Cl2O6
|
|||
| Canonical SMILES |
CCOC(=O)C1(CCC2C1(CC(C3C2CCC4=CC(=O)C=CC34C)O)C)OC(=O)C(Cl)Cl
|
|||
| InChI |
1S/C24H30Cl2O6/c1-4-31-21(30)24(32-20(29)19(25)26)10-8-16-15-6-5-13-11-14(27)7-9-22(13,2)18(15)17(28)12-23(16,24)3/h7,9,11,15-19,28H,4-6,8,10,12H2,1-3H3/t15-,16-,17-,18+,22-,23-,24-/m0/s1
|
|||
| InChIKey |
QAIOVDNCIZSSSF-RFAJLIJZSA-N
|
|||
| CAS Number |
CAS 199331-40-3
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:135783
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glucocorticoid receptor (NR3C1) | Target Info | Modulator | [2] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| NetPath Pathway | IL2 Signaling Pathway | |||
| TCR Signaling Pathway | ||||
| Pathway Interaction Database | Regulation of nuclear SMAD2/3 signaling | |||
| Signaling events mediated by HDAC Class II | ||||
| FOXA2 and FOXA3 transcription factor networks | ||||
| Glucocorticoid receptor regulatory network | ||||
| Regulation of Androgen receptor activity | ||||
| AP-1 transcription factor network | ||||
| Reactome | BMAL1:CLOCK,NPAS2 activates circadian gene expression | |||
| WikiPathways | Serotonin Receptor 4/6/7 and NR3C Signaling | |||
| SIDS Susceptibility Pathways | ||||
| Nuclear Receptors Meta-Pathway | ||||
| Endoderm Differentiation | ||||
| Hair Follicle Development: Cytodifferentiation (Part 3 of 3) | ||||
| Adipogenesis | ||||
| Circadian Clock | ||||
| Nuclear Receptors | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00035503) Multicenter Trial For Patients With Acute Crohn's Disease. U.S. National Institutes of Health. | |||
| REF 2 | Advances in corticosteroid therapy for ocular inflammation: loteprednol etabonate.Int J Inflam.2012;2012:789623. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

