Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T11615
(Former ID: TTDR01200)
|
|||||
| Target Name |
Cystine/glutamate transporter (SLC7A11)
|
|||||
| Synonyms |
XCT; X(c)-cystine transporter; X(c)- plasma membrane cystine transporter; Solute carrier family 7 member 11; Calcium channel blocker resistance protein CCBR1; Amino acid transport system xc-; Amino acid transport system XCT
Click to Show/Hide
|
|||||
| Gene Name |
SLC7A11
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Body-focused behaviour disorder [ICD-11: 6B25] | |||||
| Function |
Sodium-independent, high-affinity exchange of anionic amino acids with high specificity for anionic form of cystine and glutamate.
Click to Show/Hide
|
|||||
| BioChemical Class |
Amino acid-polyamine-organocation
|
|||||
| UniProt ID | ||||||
| Sequence |
MVRKPVVSTISKGGYLQGNVNGRLPSLGNKEPPGQEKVQLKRKVTLLRGVSIIIGTIIGA
GIFISPKGVLQNTGSVGMSLTIWTVCGVLSLFGALSYAELGTTIKKSGGHYTYILEVFGP LPAFVRVWVELLIIRPAATAVISLAFGRYILEPFFIQCEIPELAIKLITAVGITVVMVLN SMSVSWSARIQIFLTFCKLTAILIIIVPGVMQLIKGQTQNFKDAFSGRDSSITRLPLAFY YGMYAYAGWFYLNFVTEEVENPEKTIPLAICISMAIVTIGYVLTNVAYFTTINAEELLLS NAVAVTFSERLLGNFSLAVPIFVALSCFGSMNGGVFAVSRLFYVASREGHLPEILSMIHV RKHTPLPAVIVLHPLTMIMLFSGDLDSLLNFLSFARWLFIGLAVAGLIYLRYKCPDMHRP FKVPLFIPALFSFTCLFMVALSLYSDPFSTGIGFVITLTGVPAYYLFIIWDKKPRWFRIM SEKITRTLQIILEVVPEEDKL Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T43WIP | |||||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: 2-[(1S)-1-[4-[2-(4-chloranylphenoxy)ethanoyl]piperazin-1-yl]ethyl]-3-(2-ethoxyphenyl)quinazolin-4-one | Ligand Info | |||||
| Structure Description | Overall structure of Erastin-bound xCT-4F2hc complex | PDB:7EPZ | ||||
| Method | Electron microscopy | Resolution | 3.40 Å | Mutation | No | [3] |
| PDB Sequence |
TLLRGVSIII
54 GTIIGAGIFI64 SPKGVLQNTG74 SVGMSLTIWT84 VCGVLSLFGA94 LSYAELGTTI 104 KKSGGHYTYI114 LEVFGPLPAF124 VRVWVELLII134 RPAATAVISL144 AFGRYILEPF 154 FIQCEIPELA164 IKLITAVGIT174 VVMVLNSMSV184 SWSARIQIFL194 TFCKLTAILI 204 IIVPGVMQLI214 KGQTQNFKDA224 FSGRDSSITR234 LPLAFYYGMY244 AYAGWFYLNF 254 VTEEVENPEK264 TIPLAICISM274 AIVTIGYVLT284 NVAYFTTINA294 EELLLSNAVA 304 VTFSERLLGN314 FSLAVPIFVA324 LSCFGSMNGG334 VFAVSRLFYV344 ASREGHLPEI 354 LSMIHVRKHT364 PLPAVIVLHP374 LTMIMLFSGD384 LDSLLNFLSF394 ARWLFIGLAV 404 AGLIYLRYKC414 PDMHRPFKVP424 LFIPALFSFT434 CLFMVALSLY444 SDPFSTGIGF 454 VITLTGVPAY464 YLFIIWDKKP474 RWFRIMSEKI484 TRTLQIILEV494 VPE |
|||||
|
|
||||||
| Ligand Name: Distearoyl phosphatidic acid | Ligand Info | |||||
| Structure Description | Overall structure of Erastin-bound xCT-4F2hc complex | PDB:7EPZ | ||||
| Method | Electron microscopy | Resolution | 3.40 Å | Mutation | No | [3] |
| PDB Sequence |
TLLRGVSIII
54 GTIIGAGIFI64 SPKGVLQNTG74 SVGMSLTIWT84 VCGVLSLFGA94 LSYAELGTTI 104 KKSGGHYTYI114 LEVFGPLPAF124 VRVWVELLII134 RPAATAVISL144 AFGRYILEPF 154 FIQCEIPELA164 IKLITAVGIT174 VVMVLNSMSV184 SWSARIQIFL194 TFCKLTAILI 204 IIVPGVMQLI214 KGQTQNFKDA224 FSGRDSSITR234 LPLAFYYGMY244 AYAGWFYLNF 254 VTEEVENPEK264 TIPLAICISM274 AIVTIGYVLT284 NVAYFTTINA294 EELLLSNAVA 304 VTFSERLLGN314 FSLAVPIFVA324 LSCFGSMNGG334 VFAVSRLFYV344 ASREGHLPEI 354 LSMIHVRKHT364 PLPAVIVLHP374 LTMIMLFSGD384 LDSLLNFLSF394 ARWLFIGLAV 404 AGLIYLRYKC414 PDMHRPFKVP424 LFIPALFSFT434 CLFMVALSLY444 SDPFSTGIGF 454 VITLTGVPAY464 YLFIIWDKKP474 RWFRIMSEKI484 TRTLQIILEV494 VPE |
|||||
|
|
TRP128
4.122
LEU132
3.784
VAL178
4.156
SER181
4.306
MET182
3.979
GLY349
4.396
HIS350
4.160
LEU351
3.181
PRO352
3.295
GLU353
3.811
HIS359
4.449
ARG361
3.675
LEU366
3.792
VAL369
4.659
ILE370
3.779
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Ferroptosis | hsa04216 | Affiliated Target |
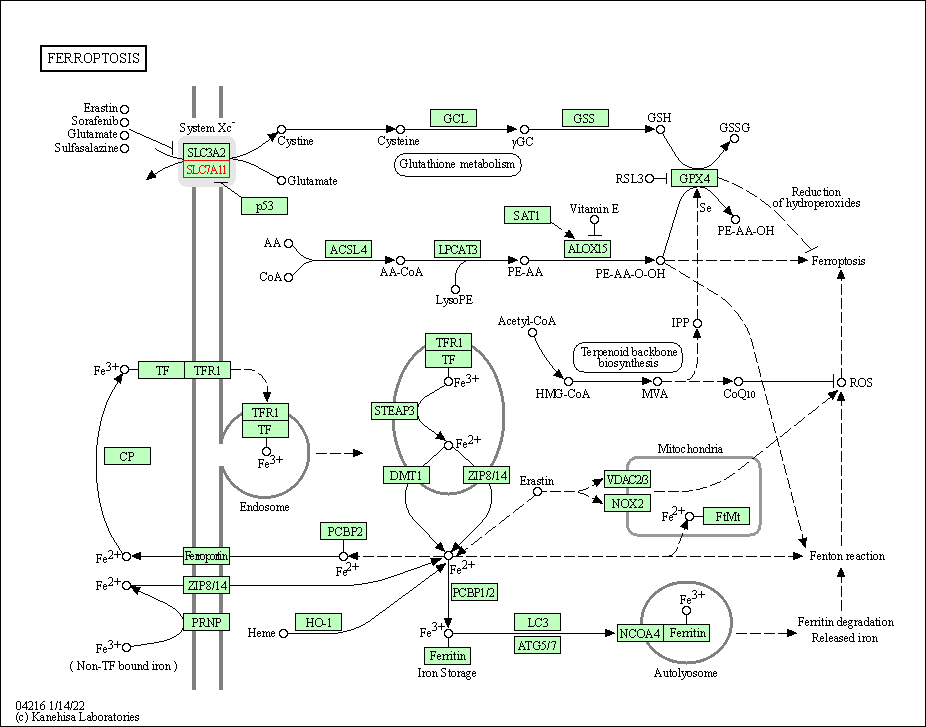
|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Degree | 2 | Degree centrality | 2.15E-04 | Betweenness centrality | 1.76E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.00E-01 | Radiality | 1.35E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 2.80E+01 | Topological coefficient | 5.09E-01 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001 Oct;15(10):1633-40. | |||||
| REF 2 | ClinicalTrials.gov (NCT03797521) A Study in Patients With Trichotillomania (TTM). U.S. National Institutes of Health. | |||||
| REF 3 | The structure of erastin-bound xCT-4F2hc complex?reveals molecular mechanisms underlying erastin-induced ferroptosis. Cell Res. 2022 Jul;32(7):687-690. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

