Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T13260
(Former ID: TTDS00252)
|
|||||
| Target Name |
Aromatase (CYP19A1)
|
|||||
| Synonyms |
P-450AROM; Estrogen synthetase; Estrogen synthase; Cytochrome P450 19A1; Cytochrome P-450AROM; CYPXIX; CYP19; CYAR; ARO1
Click to Show/Hide
|
|||||
| Gene Name |
CYP19A1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Breast cancer [ICD-11: 2C60-2C6Y] | |||||
| 2 | Cushing syndrome [ICD-11: 5A70] | |||||
| Function |
Catalyzes the formation of aromatic C18 estrogens from C19 androgens.
Click to Show/Hide
|
|||||
| BioChemical Class |
Paired donor oxygen oxidoreductase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 1.14.14.14
|
|||||
| Sequence |
MVLEMLNPIHYNITSIVPEAMPAATMPVLLLTGLFLLVWNYEGTSSIPGPGYCMGIGPLI
SHGRFLWMGIGSACNYYNRVYGEFMRVWISGEETLIISKSSSMFHIMKHNHYSSRFGSKL GLQCIGMHEKGIIFNNNPELWKTTRPFFMKALSGPGLVRMVTVCAESLKTHLDRLEEVTN ESGYVDVLTLLRRVMLDTSNTLFLRIPLDESAIVVKIQGYFDAWQALLIKPDIFFKISWL YKKYEKSVKDLKDAIEVLIAEKRRRISTEEKLEECMDFATELILAEKRGDLTRENVNQCI LEMLIAAPDTMSVSLFFMLFLIAKHPNVEEAIIKEIQTVIGERDIKIDDIQKLKVMENFI YESMRYQPVVDLVMRKALEDDVIDGYPVKKGTNIILNIGRMHRLEFFPKPNEFTLENFAK NVPYRYFQPFGFGPRGCAGKYIAMVMMKAILVTLLRRFHVKTLQGQCVESIQKIHDLSLH PDETKNMLEMIFTPRNSDRCLEH Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T88LSZ | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 4 Approved Drugs | + | ||||
| 1 | Aminoglutethimide | Drug Info | Approved | Cushing disease | [7], [8] | |
| 2 | Exemestane | Drug Info | Approved | Hormonally-responsive breast cancer | [9], [10] | |
| 3 | Letrozole | Drug Info | Approved | Hormonally-responsive breast cancer | [11], [3] | |
| 4 | Testolactone | Drug Info | Approved | Breast cancer | [12], [10] | |
| Clinical Trial Drug(s) | [+] 4 Clinical Trial Drugs | + | ||||
| 1 | Atamestane + Toremifene | Drug Info | Phase 3 | Breast cancer | [13] | |
| 2 | LIAROZOLE | Drug Info | Phase 2/3 | Dermatological disease | [14], [15] | |
| 3 | BGS-649 | Drug Info | Phase 2 | Endometriosis | [17] | |
| 4 | Dextromethorphan+quinidine | Drug Info | Phase 2 | Diabetic neuropathy | [18] | |
| Discontinued Drug(s) | [+] 4 Discontinued Drugs | + | ||||
| 1 | FORMESTANE | Drug Info | Withdrawn from market | Breast cancer | [5], [19] | |
| 2 | FINROZOLE | Drug Info | Discontinued in Phase 2 | Prostate disease | [22] | |
| 3 | MINAMESTANE | Drug Info | Terminated | Bladder cancer | [23] | |
| 4 | Rogletimide | Drug Info | Terminated | Breast cancer | [24] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 197 Inhibitor drugs | + | ||||
| 1 | Aminoglutethimide | Drug Info | [1] | |||
| 2 | Exemestane | Drug Info | [25] | |||
| 3 | Letrozole | Drug Info | [25] | |||
| 4 | Testolactone | Drug Info | [26], [27] | |||
| 5 | LIAROZOLE | Drug Info | [29] | |||
| 6 | BGS-649 | Drug Info | [30] | |||
| 7 | Dextromethorphan+quinidine | Drug Info | [31] | |||
| 8 | NARINGENIN | Drug Info | [29] | |||
| 9 | FORMESTANE | Drug Info | [32] | |||
| 10 | FINROZOLE | Drug Info | [5], [33] | |||
| 11 | VOROZOLE | Drug Info | [34] | |||
| 12 | MINAMESTANE | Drug Info | [5], [35] | |||
| 13 | Rogletimide | Drug Info | [36] | |||
| 14 | (+/-)-7-methoxy-2-(4-methoxyphenyl)chroman-4-one | Drug Info | [37] | |||
| 15 | (+/-)-7-methoxy-2-phenylchroman-4-one | Drug Info | [38] | |||
| 16 | (2S)-5,7,2',4'-tetrahydroxyflavanone | Drug Info | [39] | |||
| 17 | (2S)-abyssinone II | Drug Info | [39] | |||
| 18 | (2S)-euchrenone a7 | Drug Info | [39] | |||
| 19 | 1-(1-Benzyl-2-biphenyl-4-yl-ethyl)-1H-imidazole | Drug Info | [40] | |||
| 20 | 1-(2-(benzo[b]thiophen-4-yl)ethyl)-1H-imidazole | Drug Info | [41] | |||
| 21 | 1-(2-phenoxybenzyl)-1H-imidazole | Drug Info | [42] | |||
| 22 | 1-(3-(4-fluorophenyl)propyl)-1H-imidazole | Drug Info | [29] | |||
| 23 | 1-(3-Methoxy-naphthalen-2-yl)-1H-imidazole | Drug Info | [43] | |||
| 24 | 1-(4-Cyanobenzyl)-5-methyl-1H-imidazole | Drug Info | [44] | |||
| 25 | 1-(4-Nitro-2-phenylsulfanylbenzyl)-1H-imidazole | Drug Info | [42] | |||
| 26 | 1-(7-Methoxy-2-phenyl-chroman-4-yl)-1H-imidazole | Drug Info | [45] | |||
| 27 | 1-(9-phenyl-9H-fluoren-9-yl)-1H-1,2,4-triazole | Drug Info | [41] | |||
| 28 | 1-(9-Phenyl-9H-fluoren-9-yl)1H-imidazole | Drug Info | [29] | |||
| 29 | 1-(9H-fluoren-9-yl)-1H-imidazole | Drug Info | [41] | |||
| 30 | 1-(biphenyl-3-ylmethyl)-1H-1,2,4-triazole | Drug Info | [46] | |||
| 31 | 1-Bromo-4-imidazol-1-ylmethyl-xanthen-9-one | Drug Info | [47] | |||
| 32 | 1-Ethyl-5-(imidazol-1-yl-phenyl-methyl)-1H-indole | Drug Info | [48] | |||
| 33 | 1-Imidazol-1-ylmethyl-4-nitro-xanthen-9-one | Drug Info | [47] | |||
| 34 | 1-Imidazol-1-ylmethylxanthen-9-one | Drug Info | [42] | |||
| 35 | 1-Naphthalen-2-yl-1H-imidazole | Drug Info | [43] | |||
| 36 | 1-[(7-Fluoronaphth-2-yl)methyl]-1H-imidazole | Drug Info | [29] | |||
| 37 | 10-EPI-8-DEOXY-CUMAMBRIN B | Drug Info | [29] | |||
| 38 | 11BETA,13-DIHYDRO-10-EPI-8-DEOXYCUMAM-BRIN B | Drug Info | [29] | |||
| 39 | 2,3,4-Trimethoxy-4'-amino-trans-stilbene | Drug Info | [49] | |||
| 40 | 2,3,5-Trimethoxy-4'-amino-trans-stilbene | Drug Info | [49] | |||
| 41 | 2,3-Dimethoxy-4'-amino-trans-stilbene | Drug Info | [49] | |||
| 42 | 2,4-Dimethoxy-3'-amino-trans-stilbene | Drug Info | [49] | |||
| 43 | 2,4-Dimethoxy-4'-amino-trans-stilbene | Drug Info | [49] | |||
| 44 | 2-(1H-Imidazol-1-yl)-1-(4-nitrophenyl)ethanone | Drug Info | [49] | |||
| 45 | 2-(3-hydroxyphenyl)-7-methoxychroman-4-one | Drug Info | [38] | |||
| 46 | 2-Imidazol-1-ylmethylxanthen-9-one | Drug Info | [42] | |||
| 47 | 2-phenyl-2,3-dihydrobenzo[h]chromen-4-one | Drug Info | [38] | |||
| 48 | 2-Phenyl-3-pyridin-4-ylmethylene-chroman-4-one | Drug Info | [50] | |||
| 49 | 2-Phenyl-4-[1,2,4]triazol-1-yl-chroman-7-ol | Drug Info | [45] | |||
| 50 | 3,4'-(Ethane-1,2-diyl)dibenzenamine | Drug Info | [49] | |||
| 51 | 3,4,5-Trimethoxy-3'-amino-trans-stilbene | Drug Info | [49] | |||
| 52 | 3,4,5-Trimethoxy-4'-amino-trans-stilbene | Drug Info | [49] | |||
| 53 | 3,4-bis(3,4-dimethoxyphenyl)furan-2(5H)-one | Drug Info | [29] | |||
| 54 | 3,4-Dimethoxy-4'-amino-trans-stilbene | Drug Info | [49] | |||
| 55 | 3,5-Diacetoxy-4'-amino-trans-stilbene | Drug Info | [49] | |||
| 56 | 3,5-Diamino-4'-amino-trans-stilbene | Drug Info | [49] | |||
| 57 | 3,5-Dihydroxyl-4'-amino-trans-stilbene | Drug Info | [49] | |||
| 58 | 3,5-Dimethoxy-4'-amino-trans-stilbene | Drug Info | [49] | |||
| 59 | 3-((1H-imidazol-1-yl)methyl)-9H-xanthen-9-one | Drug Info | [42] | |||
| 60 | 3-(1-ethyl-3,4-dihydronaphthalen-2-yl)-pyridine | Drug Info | [51] | |||
| 61 | 3-(1-methyl-3,4-dihydronaphthalen-2-yl)-pyridine | Drug Info | [51] | |||
| 62 | 3-(2,2-Diphenyl-vinyl)-pyridine | Drug Info | [52] | |||
| 63 | 3-(3,4-dihydronaphthalen-2-yl)pyridine | Drug Info | [51] | |||
| 64 | 3-(3-methyl-3,4-dihydronaphthalen-2-yl)pyridine | Drug Info | [51] | |||
| 65 | 3-(4-Amino-phenyl)-1-methyl-pyrrolidine-2,5-dione | Drug Info | [53] | |||
| 66 | 3-(4-Amino-phenyl)-3-butyl-piperidine-2,6-dione | Drug Info | [54] | |||
| 67 | 3-(4-Amino-phenyl)-3-heptyl-piperidine-2,6-dione | Drug Info | [54] | |||
| 68 | 3-(4-Amino-phenyl)-3-hexyl-piperidine-2,6-dione | Drug Info | [54] | |||
| 69 | 3-(4-Amino-phenyl)-3-methyl-pyrrolidine-2,5-dione | Drug Info | [53] | |||
| 70 | 3-(4-Amino-phenyl)-3-pentyl-piperidine-2,6-dione | Drug Info | [54] | |||
| 71 | 3-(4-Amino-phenyl)-3-propyl-piperidine-2,6-dione | Drug Info | [54] | |||
| 72 | 3-(4-Amino-phenyl)-pyrrolidine-2,5-dione | Drug Info | [53] | |||
| 73 | 3-(5-Bromo-6-methoxy-naphthalen-2-yl)-pyridine | Drug Info | [43] | |||
| 74 | 3-(5-Chloro-6-methoxy-naphthalen-2-yl)-pyridine | Drug Info | [43] | |||
| 75 | 3-(6-Ethoxy-naphthalen-2-yl)-pyridine | Drug Info | [43] | |||
| 76 | 3-(6-methoxy-3,4-dihydronaphthalen-2-yl)pyridine | Drug Info | [51] | |||
| 77 | 3-(6-methoxynaphthalen-2-yl)pyridine | Drug Info | [55] | |||
| 78 | 3-(imidazolylmethyl)-4'-methoxyflavone | Drug Info | [56] | |||
| 79 | 3-(imidazolylmethyl)-4'-nitroflavone | Drug Info | [56] | |||
| 80 | 3-(imidazolylmethyl)-7-methoxy-4'-nitroflavone | Drug Info | [56] | |||
| 81 | 3-(imidazolylmethyl)flavone | Drug Info | [56] | |||
| 82 | 3-(naphthalen-2-yl)pyridine | Drug Info | [55] | |||
| 83 | 3-Amino-4'-amino-trans-stilbene | Drug Info | [49] | |||
| 84 | 3-Fluoren-9-ylidenemethyl-pyridine | Drug Info | [52] | |||
| 85 | 3-Fluoro-4'-(pyridin-4-ylmethyl)biphenyl-4-ol | Drug Info | [57] | |||
| 86 | 3-Indan-(1E)-ylidenemethyl-pyridine | Drug Info | [52] | |||
| 87 | 3-Indan-(1Z)-ylidenemethyl-pyridine | Drug Info | [52] | |||
| 88 | 3-Methoxyl-4'-amino-trans-stilbene | Drug Info | [49] | |||
| 89 | 3-Nitro-4'-nitro-trans-stilbene | Drug Info | [49] | |||
| 90 | 3-[3-Methyl-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 91 | 3-[3-Methyl-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 92 | 3-[4-Chloro-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 93 | 3-[4-Fluoro-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 94 | 3-[4-Fluoro-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 95 | 3-[4-Methyl-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 96 | 3-[4-Methyl-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 97 | 3-[5-Bromo-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 98 | 3-[5-Chloro-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 99 | 3-[5-Chloro-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 100 | 3-[5-Ethoxy-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 101 | 3-[5-Ethoxy-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 102 | 3-[5-Fluoro-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 103 | 3-[5-Methoxy-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 104 | 3-[5-Methoxy-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 105 | 3-[6-Methoxy-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 106 | 3-[6-Methyl-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 107 | 4'-(Pyridin-4-ylmethyl)biphenyl-3,4-diol | Drug Info | [57] | |||
| 108 | 4'-bromo-3-(imidazolylmethyl)-7-methoxyflavone | Drug Info | [56] | |||
| 109 | 4'-bromo-3-(imidazolylmethyl)flavone | Drug Info | [56] | |||
| 110 | 4'-cyano-3-(imidazolylmethyl)-7-methoxyflavone | Drug Info | [56] | |||
| 111 | 4'-cyano-3-(imidazolylmethyl)flavone | Drug Info | [56] | |||
| 112 | 4-((1H-imidazol-1-yl)methyl)-2H-chromen-2-one | Drug Info | [41] | |||
| 113 | 4-((1H-imidazol-1-yl)methyl)benzonitrile | Drug Info | [44] | |||
| 114 | 4-(1-Imidazol-1-yl-vinyl)-benzonitrile | Drug Info | [58] | |||
| 115 | 4-(3,4,5-Trimethoxyphenethyl)aniline | Drug Info | [49] | |||
| 116 | 4-(3,4-Dimethoxyphenethyl)aniline | Drug Info | [49] | |||
| 117 | 4-ANDROSTENE-3-17-DIONE | Drug Info | [59], [60] | |||
| 118 | 4-Bromo-1-imidazol-1-ylmethyl-xanthen-9-one | Drug Info | [47] | |||
| 119 | 4-Fluoren-9-ylidenemethyl-pyridine | Drug Info | [52] | |||
| 120 | 4-Imidazol-1-yl-2-phenyl-chroman-7-ol | Drug Info | [45] | |||
| 121 | 4-Imidazol-1-ylmethyl-1-nitro-xanthen-9-one | Drug Info | [47] | |||
| 122 | 4-Imidazol-1-ylmethyl-2-nitroxanthen-9-one | Drug Info | [42] | |||
| 123 | 4-Imidazol-1-ylmethyl-3-nitroxanthen-9-one | Drug Info | [42] | |||
| 124 | 4-Imidazol-1-ylmethylthioxanthen-9-one | Drug Info | [42] | |||
| 125 | 4-Imidazol-1-ylmethylxanthen-9-one | Drug Info | [42] | |||
| 126 | 4-Indan-(1E)-ylidenemethyl-pyridine | Drug Info | [52] | |||
| 127 | 4-Indan-(1Z)-ylidenemethyl-pyridine | Drug Info | [52] | |||
| 128 | 4-[(3'-Hydroxybiphenyl-4-yl)methyl]pyridine | Drug Info | [57] | |||
| 129 | 4-[(4'-Hydroxybiphenyl-4-yl)methyl]pyridine | Drug Info | [57] | |||
| 130 | 4-[5-Bromo-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 131 | 4-[5-Chloro-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 132 | 4-[5-Fluoro-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 133 | 4-[5-Fluoro-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 134 | 4-[5-Methoxy-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 135 | 4-[5-Methoxy-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 136 | 4-[6-Methoxy-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 137 | 4-[6-Methoxy-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 138 | 4-[6-Methyl-indan-(1E)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 139 | 4-[6-Methyl-indan-(1Z)-ylidenemethyl]-pyridine | Drug Info | [52] | |||
| 140 | 5-Indan-(1E)-ylidenemethyl-1H-imidazole | Drug Info | [61] | |||
| 141 | 5-Indan-(1Z)-ylidenemethyl-1H-imidazole | Drug Info | [61] | |||
| 142 | 5-Pyridin-3-yl-1,3-dihydro-2H-indol-2-one | Drug Info | [55] | |||
| 143 | 5-Pyridin-3-yl-2,3-dihydro-1H-inden-1-one | Drug Info | [55] | |||
| 144 | 5-[5-Bromo-indan-(1E)-ylidenemethyl]-1H-imidazole | Drug Info | [61] | |||
| 145 | 5-[5-Bromo-indan-(1Z)-ylidenemethyl]-1H-imidazole | Drug Info | [61] | |||
| 146 | 5-[5-Fluoro-indan-(1E)-ylidenemethyl]-pyrimidine | Drug Info | [52] | |||
| 147 | 5-[5-Fluoro-indan-(1Z)-ylidenemethyl]-pyrimidine | Drug Info | [52] | |||
| 148 | 5-[5-Methoxy-indan-(1E)-ylidenemethyl]-thiazole | Drug Info | [52] | |||
| 149 | 6-((1H-imidazol-1-yl)methyl)-2H-chromene-2-thione | Drug Info | [41] | |||
| 150 | 6-Imidazol-1-yl-isoquinoline | Drug Info | [41] | |||
| 151 | 7,4'-Dihydroxyflavone | Drug Info | [29] | |||
| 152 | 7-((1H-imidazol-1-yl)methyl)-2H-chromen-2-one | Drug Info | [41] | |||
| 153 | 7-((1H-imidazol-1-yl)methyl)-4H-chromen-4-one | Drug Info | [41] | |||
| 154 | 7-((1H-imidazol-1-yl)methyl)isoquinoline | Drug Info | [41] | |||
| 155 | 7-(2-(1H-imidazol-1-yl)ethoxy)-2H-chromen-2-one | Drug Info | [62] | |||
| 156 | 7-hydroxy-2-phenylchroman-4-one | Drug Info | [38] | |||
| 157 | 7-[1,2,4]Triazol-4-ylmethyl-chromen-4-one | Drug Info | [47] | |||
| 158 | ALBANOL A | Drug Info | [39] | |||
| 159 | ALPHA-NAPHTHOFLAVONE | Drug Info | [29] | |||
| 160 | APIGENIN | Drug Info | [29] | |||
| 161 | Benzyl-biphenyl-4-ylmethyl-imidazol-1-yl-amine | Drug Info | [40] | |||
| 162 | biochanin A | Drug Info | [29] | |||
| 163 | Chrysin | Drug Info | [29] | |||
| 164 | flavone | Drug Info | [29] | |||
| 165 | Gamma-mangostin | Drug Info | [63] | |||
| 166 | GARCINONE D | Drug Info | [63] | |||
| 167 | Isogemichalcone C | Drug Info | [39] | |||
| 168 | ISOLICOFLAVONOL | Drug Info | [39] | |||
| 169 | LIQUIRTIGENIN | Drug Info | [64] | |||
| 170 | LUDARTIN | Drug Info | [29] | |||
| 171 | MDL-18962 | Drug Info | [65] | |||
| 172 | MONODICTYOCHROMONE B | Drug Info | [66] | |||
| 173 | MR-16089 | Drug Info | [29] | |||
| 174 | MR-20492 | Drug Info | [29] | |||
| 175 | MR-20494 | Drug Info | [29] | |||
| 176 | MR-20496 | Drug Info | [67] | |||
| 177 | MR-20814 | Drug Info | [67] | |||
| 178 | N-(2-benzyloxy-4-nitrophenyl)methanesulfonamide | Drug Info | [68] | |||
| 179 | N-(2-hexyloxy-4-nitrophenyl)methanesulfonamide | Drug Info | [69] | |||
| 180 | N-(2-nonyloxy-4-nitrophenyl)methanesulfonamide | Drug Info | [69] | |||
| 181 | N-(2-Propyloxy-4-nitrophenyl)methanesulfonamide | Drug Info | [69] | |||
| 182 | N-[2-(4'-Nitrophenyl)ethyl]-imidazole | Drug Info | [29] | |||
| 183 | NSC-122427 | Drug Info | [70] | |||
| 184 | NSC-12999 | Drug Info | [41] | |||
| 185 | NSC-131736 | Drug Info | [41] | |||
| 186 | NSC-289311 | Drug Info | [41] | |||
| 187 | NSC-356483 | Drug Info | [41] | |||
| 188 | NSC-356781 | Drug Info | [41] | |||
| 189 | NSC-368272 | Drug Info | [41] | |||
| 190 | NSC-368280 | Drug Info | [41] | |||
| 191 | NSC-613604 | Drug Info | [70] | |||
| 192 | NSC-666292 | Drug Info | [41] | |||
| 193 | NSC-683634 | Drug Info | [41] | |||
| 194 | NSC-75308 | Drug Info | [41] | |||
| 195 | NSC-93358 | Drug Info | [70] | |||
| 196 | NSC-94258 | Drug Info | [29] | |||
| 197 | NSC-94891 | Drug Info | [70] | |||
| Modulator | [+] 1 Modulator drugs | + | ||||
| 1 | Atamestane + Toremifene | Drug Info | [28] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Testosterone | Ligand Info | |||||
| Structure Description | HUMAN PLACENTAL AROMATASE CYTOCHROME P450 (CYP19A1) COMPLEXED WITH TESTOSTERONE | PDB:5JKW | ||||
| Method | X-ray diffraction | Resolution | 3.00 Å | Mutation | No | [71] |
| PDB Sequence |
SSIPGPGYCM
54 GIGPLISHGR64 FLWMGIGSAC74 NYYNRVYGEF84 MRVWISGEET94 LIISKSSSMF 104 HIMKHNHYSS114 RFGSKLGLQC124 IGMHEKGIIF134 NNNPELWKTT144 RPFFMKALSG 154 PGLVRMVTVC164 AESLKTHLDR174 LEEVTNESGY184 VDVLTLLRRV194 MLDTSNTLFL 204 RIPLDESAIV214 VKIQGYFDAW224 QALLIKPDIF234 FKISWLYKKY244 EKSVKDLKDA 254 IEVLIAEKRR264 RISTEEKLEE274 CMDFATELIL284 AEKRGDLTRE294 NVNQCILEML 304 IAAPDTMSVS314 LFFMLFLIAK324 HPNVEEAIIK334 EIQTVIGERD344 IKIDDIQKLK 354 VMENFIYESM364 RYQPVVDLVM374 RKALEDDVID384 GYPVKKGTNI394 ILNIGRMHRL 404 EFFPKPNEFT414 LENFAKNVPY424 RYFQPFGFGP434 RGCAGKYIAM444 VMMKAILVTL 454 LRRFHVKTLQ464 GQCVESIQKI474 HDLSLHPDET484 KNMLEMIFTP494 RN |
|||||
|
|
||||||
| Ligand Name: Exemestane | Ligand Info | |||||
| Structure Description | Crystal structure of human placental aromatase complexed with breast cancer drug exemestane | PDB:3S7S | ||||
| Method | X-ray diffraction | Resolution | 3.21 Å | Mutation | No | [72] |
| PDB Sequence |
SSIPGPGYCM
54 GIGPLISHGR64 FLWMGIGSAC74 NYYNRVYGEF84 MRVWISGEET94 LIISKSSSMF 104 HIMKHNHYSS114 RFGSKLGLQC124 IGMHEKGIIF134 NNNPELWKTT144 RPFFMKALSG 154 PGLVRMVTVC164 AESLKTHLDR174 LEEVTNESGY184 VDVLTLLRRV194 MLDTSNTLFL 204 RIPLDESAIV214 VKIQGYFDAW224 QALLIKPDIF234 FKISWLYKKY244 EKSVKDLKDA 254 IEVLIAEKRR264 RISTEEKLEE274 CMDFATELIL284 AEKRGDLTRE294 NVNQCILEML 304 IAAPDTMSVS314 LFFMLFLIAK324 HPNVEEAIIK334 EIQTVIGERD344 IKIDDIQKLK 354 VMENFIYESM364 RYQPVVDLVM374 RKALEDDVID384 GYPVKKGTNI394 ILNIGRMHRL 404 EFFPKPNEFT414 LENFAKNVPY424 RYFQPFGFGP434 RGCAGKYIAM444 VMMKAILVTL 454 LRRFHVKTLQ464 GQCVESIQKI474 HDLSLHPDET484 KNMLEMIFTP494 RN |
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Steroid hormone biosynthesis | hsa00140 | Affiliated Target |

|
| Class: Metabolism => Lipid metabolism | Pathway Hierarchy | ||
| Ovarian steroidogenesis | hsa04913 | Affiliated Target |
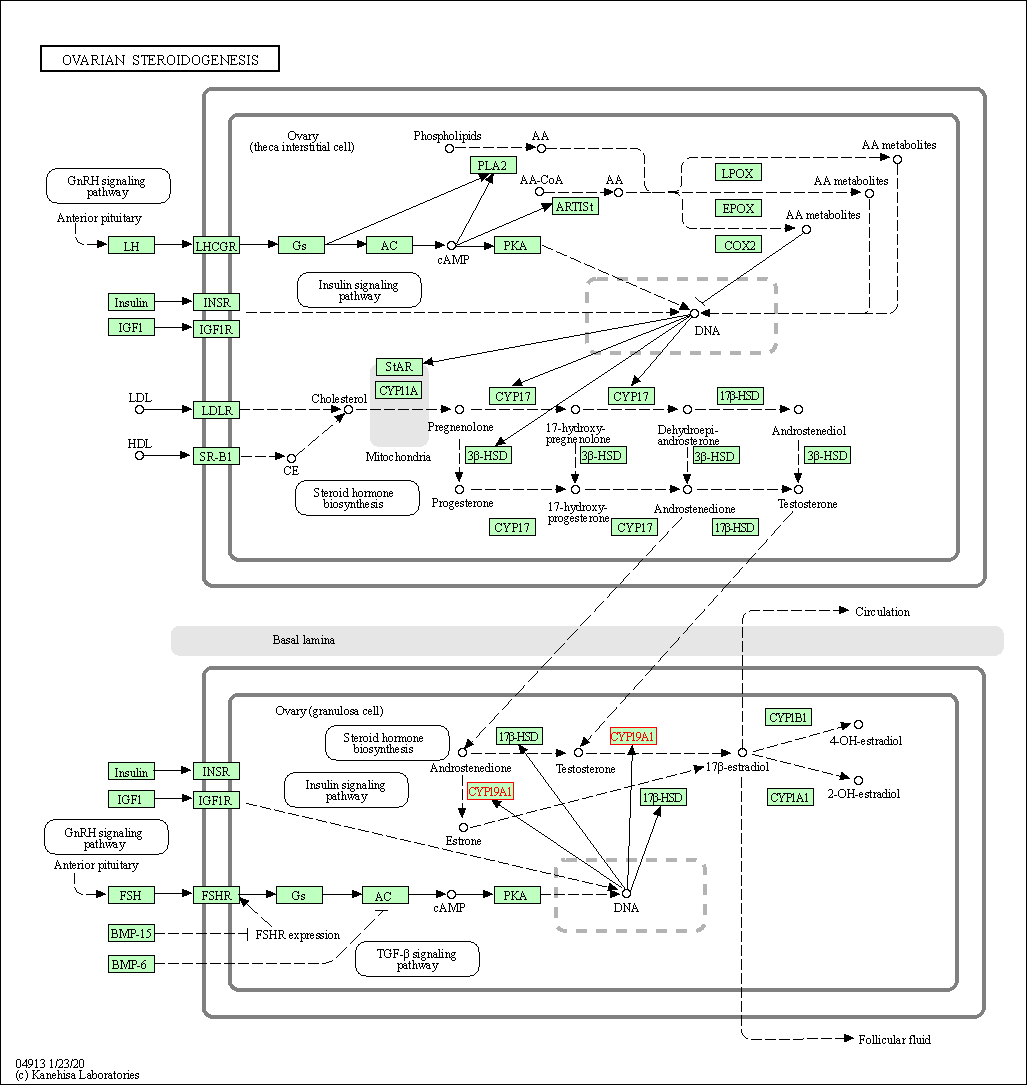
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 12 | Degree centrality | 1.29E-03 | Betweenness centrality | 2.99E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.62E-01 | Radiality | 1.24E+01 | Clustering coefficient | 5.00E-01 |
| Neighborhood connectivity | 1.08E+01 | Topological coefficient | 2.76E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-regulating Transcription Factors | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| BioCyc | [+] 3 BioCyc Pathways | + | ||||
| 1 | Superpathway of steroid hormone biosynthesis | |||||
| 2 | Estradiol biosynthesis II | |||||
| 3 | Estradiol biosynthesis I | |||||
| KEGG Pathway | [+] 3 KEGG Pathways | + | ||||
| 1 | Steroid hormone biosynthesis | |||||
| 2 | Metabolic pathways | |||||
| 3 | Ovarian steroidogenesis | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | FSH Signaling Pathway | |||||
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | Androgen/estrogene/progesterone biosynthesis | |||||
| Pathwhiz Pathway | [+] 1 Pathwhiz Pathways | + | ||||
| 1 | Androgen and Estrogen Metabolism | |||||
| Reactome | [+] 1 Reactome Pathways | + | ||||
| 1 | Endogenous sterols | |||||
| WikiPathways | [+] 8 WikiPathways | + | ||||
| 1 | Metapathway biotransformation | |||||
| 2 | Tryptophan metabolism | |||||
| 3 | Oxidation by Cytochrome P450 | |||||
| 4 | Ovarian Infertility Genes | |||||
| 5 | Metabolism of steroid hormones and vitamin D | |||||
| 6 | FSH signaling pathway | |||||
| 7 | Integrated Breast Cancer Pathway | |||||
| 8 | Phase 1 - Functionalization of compounds | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Aminoglutethimide-induced protein free radical formation on myeloperoxidase: a potential mechanism of agranulocytosis. Chem Res Toxicol. 2007 Jul;20(7):1038-45. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5137). | |||||
| REF 3 | New drugs in development for the treatment of endometriosis. Expert Opin Investig Drugs. 2008 Aug;17(8):1187-202. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8311). | |||||
| REF 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 6 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000824) | |||||
| REF 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7054). | |||||
| REF 8 | Breakdown of Th cell immune responses and steroidogenic CYP11A1 expression in CD4+ T cells in a murine model implanted with B16 melanoma. Cell Immunol. 2000 Nov 25;206(1):7-15. | |||||
| REF 9 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7073). | |||||
| REF 10 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5209). | |||||
| REF 12 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7303). | |||||
| REF 13 | ClinicalTrials.gov (NCT00044291) Phase III Study of Atamestane Plus Toremifene Versus Letrozole in Advanced Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 14 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5210). | |||||
| REF 15 | ClinicalTrials.gov (NCT00282724) Efficacy and Safety of Two Doses of Liarozole vs. Placebo for the Treatment of Lamellar Ichthyosis. U.S. National Institutes of Health. | |||||
| REF 16 | Irosustat: a first-generation steroid sulfatase inhibitor in breast cancer. Expert Rev Anticancer Ther. 2011 Feb;11(2):179-83. | |||||
| REF 17 | ClinicalTrials.gov (NCT01190475) BGS649 Monotherapy in Moderate to Severe Endometriosis Patients. U.S. National Institutes of Health. | |||||
| REF 18 | Dextromethorphan/quinidine: AVP 923, dextromethorphan/cytochrome P450-2D6 inhibitor, quinidine/dextromethorphan. Drugs R D. 2005;6(3):174-7. | |||||
| REF 19 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000733) | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003076) | |||||
| REF 21 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002721) | |||||
| REF 22 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010074) | |||||
| REF 23 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002545) | |||||
| REF 24 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001207) | |||||
| REF 25 | Aromatase inhibitors--theoretical concept and present experiences in the treatment of endometriosis. Zentralbl Gynakol. 2003 Jul-Aug;125(7-8):247-51. | |||||
| REF 26 | Aromatase inhibitors for male infertility. J Urol. 2002 Feb;167(2 Pt 1):624-9. | |||||
| REF 27 | Testosterone versus testosterone and testolactone in treating reproductive and sexual dysfunction in men with epilepsy and hypogonadism. Neurology. 1998 Mar;50(3):782-4. | |||||
| REF 28 | Toremifene-atamestane; alone or in combination: predictions from the preclinical intratumoral aromatase model. J Steroid Biochem Mol Biol. 2008 Jan;108(1-2):1-7. | |||||
| REF 29 | Pharmacophore modeling strategies for the development of novel nonsteroidal inhibitors of human aromatase (CYP19). Bioorg Med Chem Lett. 2010 May 15;20(10):3050-64. | |||||
| REF 30 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800032111) | |||||
| REF 31 | Emerging drugs in neuropathic pain. Expert Opin Emerg Drugs. 2007 Mar;12(1):113-26. | |||||
| REF 32 | The taiwaniaquinoids: a review. J Nat Prod. 2010 Feb 26;73(2):284-98. | |||||
| REF 33 | Pharmacokinetics of finrozole (MPV-2213ad), a novel selective aromatase inhibitor, in healthy men. Br J Clin Pharmacol. 2001 Dec;52(6):702-4. | |||||
| REF 34 | Chiral aromatase and dual aromatase-steroid sulfatase inhibitors from the letrozole template: synthesis, absolute configuration, and in vitro activ... J Med Chem. 2008 Jul 24;51(14):4226-38. | |||||
| REF 35 | High-performance liquid chromatographic determination of FCE 24928, a new aromatase inhibitor, in human plasma. J Chromatogr A. 1994 Feb 4;660(1-2):293-8. | |||||
| REF 36 | Analogues of 3-ethyl-3-(4-pyridyl)piperidine-2,6-dione as selective inhibitors of aromatase: derivatives with variable 1-alkyl and 3-alkyl substitu... J Med Chem. 1987 Sep;30(9):1550-4. | |||||
| REF 37 | Synthesis and biological evaluation of (+/-)-abyssinone II and its analogues as aromatase inhibitors for chemoprevention of breast cancer. J Med Chem. 2007 Jun 14;50(12):2799-806. | |||||
| REF 38 | New 7,8-benzoflavanones as potent aromatase inhibitors: synthesis and biological evaluation. Bioorg Med Chem. 2008 Feb 1;16(3):1474-80. | |||||
| REF 39 | Aromatase inhibitors from Broussonetia papyrifera. J Nat Prod. 2001 Oct;64(10):1286-93. | |||||
| REF 40 | CYP19 (aromatase): exploring the scaffold flexibility for novel selective inhibitors. Bioorg Med Chem. 2008 Sep 15;16(18):8349-58. | |||||
| REF 41 | Fast three dimensional pharmacophore virtual screening of new potent non-steroid aromatase inhibitors. J Med Chem. 2009 Jan 8;52(1):143-50. | |||||
| REF 42 | Novel highly potent and selective nonsteroidal aromatase inhibitors: synthesis, biological evaluation and structure-activity relationships investigation. J Med Chem. 2010 Jul 22;53(14):5347-51. | |||||
| REF 43 | Heteroaryl-substituted naphthalenes and structurally modified derivatives: selective inhibitors of CYP11B2 for the treatment of congestive heart fa... J Med Chem. 2005 Oct 20;48(21):6632-42. | |||||
| REF 44 | Fadrozole hydrochloride: a potent, selective, nonsteroidal inhibitor of aromatase for the treatment of estrogen-dependent disease. J Med Chem. 1991 Feb;34(2):725-36. | |||||
| REF 45 | Synthesis and evaluation of 4-triazolylflavans as new aromatase inhibitors. Bioorg Med Chem Lett. 2004 Oct 18;14(20):5215-8. | |||||
| REF 46 | Highly potent first examples of dual aromatase-steroid sulfatase inhibitors based on a biphenyl template. J Med Chem. 2010 Mar 11;53(5):2155-70. | |||||
| REF 47 | A new class of nonsteroidal aromatase inhibitors: design and synthesis of chromone and xanthone derivatives and inhibition of the P450 enzymes aromatase and 17 alpha-hydroxylase/C17,20-lyase. J Med Chem. 2001 Mar 1;44(5):672-80. | |||||
| REF 48 | New selective nonsteroidal aromatase inhibitors: synthesis and inhibitory activity of 2,3 or 5-(alpha-azolylbenzyl)-1H-indoles. Bioorg Med Chem Lett. 1999 Feb 8;9(3):333-6. | |||||
| REF 49 | Design, synthesis, and biological evaluation of resveratrol analogues as aromatase and quinone reductase 2 inhibitors for chemoprevention of cancer. Bioorg Med Chem. 2010 Jul 15;18(14):5352-66. | |||||
| REF 50 | New aromatase inhibitors. Synthesis and inhibitory activity of pyridinyl-substituted flavanone derivatives. Bioorg Med Chem Lett. 2002 Apr 8;12(7):1059-61. | |||||
| REF 51 | Synthesis and evaluation of heteroaryl-substituted dihydronaphthalenes and indenes: potent and selective inhibitors of aldosterone synthase (CYP11B... J Med Chem. 2006 Apr 6;49(7):2222-31. | |||||
| REF 52 | Synthesis and evaluation of (pyridylmethylene)tetrahydronaphthalenes/-indanes and structurally modified derivatives: potent and selective inhibitor... J Med Chem. 2005 Mar 10;48(5):1563-75. | |||||
| REF 53 | Synthesis and biochemical evaluation of analogues of aminoglutethimide based on phenylpyrrolidine-2,5-dione. J Med Chem. 1986 Apr;29(4):520-3. | |||||
| REF 54 | Aromatase inhibitors. Synthesis and evaluation of mammary tumor inhibiting activity of 3-alkylated 3-(4-aminophenyl)piperidine-2,6-diones. J Med Chem. 1986 Aug;29(8):1362-9. | |||||
| REF 55 | In vivo active aldosterone synthase inhibitors with improved selectivity: lead optimization providing a series of pyridine substituted 3,4-dihydro-... J Med Chem. 2008 Dec 25;51(24):8077-87. | |||||
| REF 56 | Lead optimization providing a series of flavone derivatives as potent nonsteroidal inhibitors of the cytochrome P450 aromatase enzyme. J Med Chem. 2006 Jul 27;49(15):4777-80. | |||||
| REF 57 | Replacement of imidazolyl by pyridyl in biphenylmethylenes results in selective CYP17 and dual CYP17/CYP11B1 inhibitors for the treatment of prosta... J Med Chem. 2010 Aug 12;53(15):5749-58. | |||||
| REF 58 | Aromatase inhibitors: synthesis, biological activity, and binding mode of azole-type compounds. J Med Chem. 1993 May 14;36(10):1393-400. | |||||
| REF 59 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 60 | Synthesis of androst-5-en-7-ones and androsta-3,5-dien-7-ones and their related 7-deoxy analogs as conformational and catalytic probes for the acti... J Med Chem. 1994 Jul 8;37(14):2198-205. | |||||
| REF 61 | Synthesis and evaluation of imidazolylmethylenetetrahydronaphthalenes and imidazolylmethyleneindanes: potent inhibitors of aldosterone synthase. J Med Chem. 2005 Mar 24;48(6):1796-805. | |||||
| REF 62 | Design, synthesis, and 3D QSAR of novel potent and selective aromatase inhibitors. J Med Chem. 2004 Dec 30;47(27):6792-803. | |||||
| REF 63 | Xanthones from the botanical dietary supplement mangosteen (Garcinia mangostana) with aromatase inhibitory activity. J Nat Prod. 2008 Jul;71(7):1161-6. | |||||
| REF 64 | Screening of herbal constituents for aromatase inhibitory activity. Bioorg Med Chem. 2008 Sep 15;16(18):8466-70. | |||||
| REF 65 | 6 beta-Propynyl-substituted steroids: mechanism-based enzyme-activated irreversible inhibitors of aromatase. J Med Chem. 1997 Sep 26;40(20):3263-70. | |||||
| REF 66 | Monodictyochromes A and B, dimeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis. J Nat Prod. 2008 Nov;71(11):1793-9. | |||||
| REF 67 | Design and synthesis of a new type of non steroidal human aromatase inhibitors. Bioorg Med Chem Lett. 1998 May 5;8(9):1041-4. | |||||
| REF 68 | Synthesis and biological evaluation of selective aromatase expression regulators in breast cancer cells. J Med Chem. 2007 Apr 5;50(7):1635-44. | |||||
| REF 69 | Novel sulfonanilide analogues suppress aromatase expression and activity in breast cancer cells independent of COX-2 inhibition. J Med Chem. 2006 Feb 23;49(4):1413-9. | |||||
| REF 70 | An efficient steroid pharmacophore-based strategy to identify new aromatase inhibitors. Eur J Med Chem. 2009 Oct;44(10):4121-7. | |||||
| REF 71 | Testosterone complex and non-steroidal ligands of human aromatase. J Steroid Biochem Mol Biol. 2018 Jul;181:11-19. | |||||
| REF 72 | Novel aromatase inhibitors by structure-guided design. J Med Chem. 2012 Oct 11;55(19):8464-76. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

