Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T14755
(Former ID: TTDR00284)
|
|||||
| Target Name |
TNF related activation protein (CD40LG)
|
|||||
| Synonyms |
Tumor necrosis factor ligand superfamily member 5; TRAP; TNFSF5; TNF-related activation protein; T-cell antigen Gp39; T cell antigen Gp39; CD40L; CD40-L; CD40 ligand; CD154 antigen; CD154
Click to Show/Hide
|
|||||
| Gene Name |
CD40LG
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 11 Target-related Diseases | + | ||||
| 1 | Diabetes mellitus [ICD-11: 5A10] | |||||
| 2 | Lupus erythematosus [ICD-11: 4A40] | |||||
| 3 | Lymphoma [ICD-11: 2A80-2A86] | |||||
| 4 | Mature B-cell leukaemia [ICD-11: 2A82] | |||||
| 5 | Mature B-cell lymphoma [ICD-11: 2A85] | |||||
| 6 | B-cell lymphoma [ICD-11: 2A86] | |||||
| 7 | Prostate cancer [ICD-11: 2C82] | |||||
| 8 | Rheumatoid arthritis [ICD-11: FA20] | |||||
| 9 | Sjogren syndrome [ICD-11: 4A43] | |||||
| 10 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| 11 | Thrombocytopenia [ICD-11: 3B64] | |||||
| Function |
Costimulates T-cell proliferation and cytokine production. Its cross-linking on T-cells generates a costimulatory signal which enhances the production of IL4 and IL10 in conjunction with the TCR/CD3 ligation and CD28 costimulation. Induces the activation of NF-kappa-B and kinases MAPK8 and PAK2 in T-cells. Induces tyrosine phosphorylation of isoform 3 of CD28. Mediates B-cell proliferation in the absence of co-stimulus as well as IgE production in the presence of IL4. Involved in immunoglobulin class switching. Cytokine that binds to CD40/TNFRSF5.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine: tumor necrosis factor
|
|||||
| UniProt ID | ||||||
| Sequence |
MIETYNQTSPRSAATGLPISMKIFMYLLTVFLITQMIGSALFAVYLHRRLDKIEDERNLH
EDFVFMKTIQRCNTGERSLSLLNCEEIKSQFEGFVKDIMLNKEETKKENSFEMQKGDQNP QIAAHVISEASSKTTSVLQWAEKGYYTMSNNLVTLENGKQLTVKRQGLYYIYAQVTFCSN REASSQAPFIASLCLKSPGRFERILLRAANTHSSAKPCGQQSIHLGGVFELQPGASVFVN VTDPSQVSHGTGFTSFGLLKL Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T04TWI | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 12 Clinical Trial Drugs | + | ||||
| 1 | BI 655064 | Drug Info | Phase 2 | Lupus | [2] | |
| 2 | Dapirolizumab pegol | Drug Info | Phase 2 | Systemic lupus erythematosus | [2] | |
| 3 | IL-2/CD40L-expressing leukemia vaccine | Drug Info | Phase 2 | Chronic lymphocytic leukaemia | [3] | |
| 4 | ISF35 | Drug Info | Phase 2 | Small lymphocytic lymphoma | [4] | |
| 5 | Lucatumumab | Drug Info | Phase 2 | Lymphoma | [5] | |
| 6 | PG-102 | Drug Info | Phase 2 | Autoimmune diabetes | [6] | |
| 7 | ABBV-428 | Drug Info | Phase 1 | Solid tumour/cancer | [7], [8] | |
| 8 | BPX-101 | Drug Info | Phase 1 | Prostate cancer | [9] | |
| 9 | CDP-7657 | Drug Info | Phase 1 | Systemic lupus erythematosus | [10] | |
| 10 | Chi Lob 7/4 | Drug Info | Phase 1 | B-cell lymphoma | [11] | |
| 11 | MEDI4920 | Drug Info | Phase 1 | Sjogren syndrome | [12] | |
| 12 | UltraCD40L | Drug Info | Phase 1 | Systemic lupus erythematosus | [13] | |
| Discontinued Drug(s) | [+] 2 Discontinued Drugs | + | ||||
| 1 | IDEC-131 | Drug Info | Discontinued in Phase 1 | Thrombocytopenia | [14] | |
| 2 | Dacetuzumab | Drug Info | Terminated | Non-hodgkin lymphoma | [16] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Inhibitor | [+] 2 Inhibitor drugs | + | ||||
| 1 | Dapirolizumab pegol | Drug Info | [2] | |||
| 2 | MEDI4920 | Drug Info | [12], [25] | |||
| Binder | [+] 1 Binder drugs | + | ||||
| 1 | ISF35 | Drug Info | [18] | |||
| Modulator | [+] 5 Modulator drugs | + | ||||
| 1 | Lucatumumab | Drug Info | [19], [20] | |||
| 2 | PG-102 | Drug Info | [21] | |||
| 3 | Chi Lob 7/4 | Drug Info | [20] | |||
| 4 | UltraCD40L | Drug Info | [26] | |||
| 5 | Dacetuzumab | Drug Info | [20], [28] | |||
| Stimulator | [+] 1 Stimulator drugs | + | ||||
| 1 | ABBV-428 | Drug Info | [8] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: (S)-2-Amino-4-(7-hydroxy-2-oxo-2H-chromen-4-YL)butanoic acid | Ligand Info | |||||
| Structure Description | Crystal Structure of the Fab fragment of humanized 5c8 antibody containing the fluorescent non-canonical amino acid L-(7-hydroxycoumarin-4-yl)ethylglycine in complex with CD40L at pH 6.8 | PDB:6W9G | ||||
| Method | X-ray diffraction | Resolution | 1.82 Å | Mutation | No | [30] |
| PDB Sequence |
NPQIAAHVIS
128 EASSKTSVLQ139 WAEKGYYTMS149 NNLVTLENGK159 QLTVKRQGLY169 YIYAQVTFCS 179 NRAPFIASLC194 LKSPGRFERI204 LLRAANTHSS214 AKPCGQQSIH224 LGGVFELQPG 234 ASVFVNVTDP244 SQVSHGTGFT254 SFGLLKL
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: 2,5,8,11-Tetraoxatridecan-13-ol | Ligand Info | |||||
| Structure Description | Crystal Structure of the Fab fragment of humanized 5c8 antibody containing the fluorescent non-canonical amino acid L-(7-hydroxycoumarin-4-yl)ethylglycine in complex with CD40L at pH 6.8 | PDB:6W9G | ||||
| Method | X-ray diffraction | Resolution | 1.82 Å | Mutation | No | [30] |
| PDB Sequence |
NPQIAAHVIS
128 EASSKTSVLQ139 WAEKGYYTMS149 NNLVTLENGK159 QLTVKRQGLY169 YIYAQVTFCS 179 NRAPFIASLC194 LKSPGRFERI204 LLRAANTHSS214 AKPCGQQSIH224 LGGVFELQPG 234 ASVFVNVTDP244 SQVSHGTGFT254 SFGLLKL
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| NF-kappa B signaling pathway | hsa04064 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Cell adhesion molecules | hsa04514 | Affiliated Target |
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| T cell receptor signaling pathway | hsa04660 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Intestinal immune network for IgA production | hsa04672 | Affiliated Target |
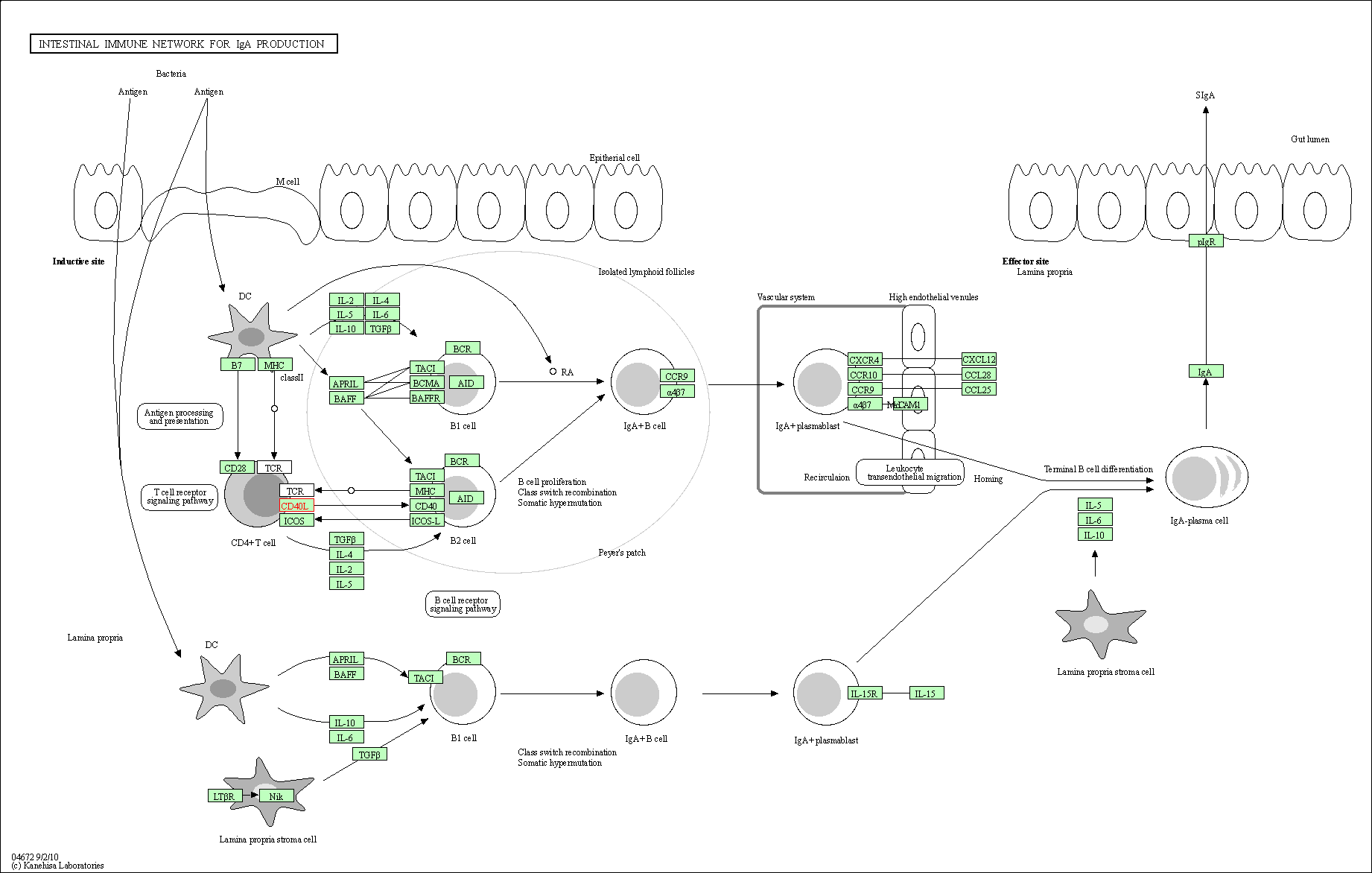
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 9 | Degree centrality | 9.67E-04 | Betweenness centrality | 2.19E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.27E-01 | Radiality | 1.40E+01 | Clustering coefficient | 5.00E-01 |
| Neighborhood connectivity | 4.58E+01 | Topological coefficient | 1.85E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Immunotherapy of high-risk acute leukemia with a recipient (autologous) vaccine expressing transgenic human CD40L and IL-2 after chemotherapy and a... Blood. 2006 Feb 15;107(4):1332-41. | |||||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 3 | ClinicalTrials.gov (NCT01604031) Treatment of B-CLL With Autologous IL2 and CD40 Ligand-Expressing Tumor Cells + Lenalidomide. U.S. National Institutes of Health. | |||||
| REF 4 | ClinicalTrials.gov (NCT00942409) Study of Repeat Intranodal Injection of Memgen's Cancer Vaccine, Ad-ISF35, in Subjects With Non-Hodgkin's Lymphoma (Follicular, Diffuse Large Cell, Mantle Cell, and Small Lymphocytic Lymphoma/Chronic Lymphocytic Leukemia. U.S. National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT00231166) Safety, Efficacy, Dose-finding Study of a Monoclonal Antibody in Patients With Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT01106703) The Effects of PG102, a Water Soluble Extract From Actinidia Arguta, on Serum Total IgE Levels. U.S. National Institutes of Health. | |||||
| REF 7 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 8 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 9 | ClinicalTrials.gov (NCT00868595) MTD Study of Vaccine BP-GMAX-CD1 Plus AP1903 to Treat Castrate Resistant Prostate Cancer. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT01093911) Safety Study of CDP7657 in Healthy Volunteers and Patients With Systemic Lupus Erythematosus (SLE). U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT01561911) A Phase I Study of the Chimeric Anti-CD40 Monoclonal Antibody ChiLob 7/4 to Treat Advanced Malignancies Refractory to Conventional Anti-cancer Treatment. U.S. National Institutes of Health. | |||||
| REF 12 | ClinicalTrials.gov (NCT02151110) A Phase 1, Randomized, Blinded, Placebo-controlled, Single-ascending Dose Study to Evaluate the Safety and Tolerability of MEDI4920 in Healthy Adults. U.S. National Institutes of Health. | |||||
| REF 13 | Soluble multi-trimeric TNF superfamily ligand adjuvants enhance immune responses to a HIV-1 Gag DNA vaccine. Vaccine. 2012 January 17; 30(4): 691-702. | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007783) | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010330) | |||||
| REF 16 | New developments in immunosuppressive therapy for heart transplantation. Expert Opin Emerg Drugs. 2009 Mar;14(1):1-21. | |||||
| REF 17 | FRI0167 Patient-Reported Outcomes (PROS) During Treatment with Mavrilimumab, A Fully Human Monoclonal Antibody Targeting GMCSFR-Alpha, In the Phase IIB Earth Explorer 1 Study | |||||
| REF 18 | Intratumoral injection of Ad-ISF35 (Chimeric CD154) breaks tolerance and induces lymphoma tumor regression. Hum Gene Ther. 2015 Jan;26(1):14-25. | |||||
| REF 19 | Phase I study of the anti-CD40 humanized monoclonal antibody lucatumumab (HCD122) in relapsed chronic lymphocytic leukemia.Leuk Lymphoma.2012 Nov;53(11):2136-42. | |||||
| REF 20 | Agonistic CD40 antibodies and cancer therapy | |||||
| REF 21 | Induction of an altered CD40 signaling complex by an antagonistic human monoclonal antibody to CD40.J Immunol.2015 May 1;194(9):4319-27. | |||||
| REF 22 | Activation of antigen-exposed iMC-DCs at the ight place and ight time promotes potent anti-tumor immunity. Oncoimmunology. 2012 May 1; 1(3): 362-363. | |||||
| REF 23 | CDP7657, an anti-CD40L antibody lacking an Fc domain, inhibits CD40L-dependent immune responses without thrombotic complications: an in vivo study. Arthritis Res Ther. 2015 Sep 3;17:234. | |||||
| REF 24 | First-in-human trial of the safety, pharmacokinetics and immunogenicity of a PEGylated anti-CD40L antibody fragment (CDP7657) in healthy individuals and patients with systemic lupus erythematosus. Lupus. 2015 Sep;24(10):1045-56. | |||||
| REF 25 | Therapeutic target database update 2012: a resource for facilitating target-oriented drug discovery. Nucleic Acids Res. 2012 Jan;40(Database issue):D1128-36. | |||||
| REF 26 | CD40L-Tri, a novel formulation of recombinant human CD40L that effectively activates B cells. Cancer Immunol Immunother. 2013 February; 62(2): 347-357. | |||||
| REF 27 | Phase I clinical trial of a monoclonal antibody against CD40-ligand (IDEC-131) in patients with systemic lupus erythematosus. J Rheumatol. 2001 Jan;28(1):95-101. | |||||
| REF 28 | A phase I study of dacetuzumab (SGN-40, a humanized anti-CD40 monoclonal antibody) in patients with chronic lymphocytic leukemia.Leuk Lymphoma.2010 Feb;51(2):228-35. | |||||
| REF 29 | Characterization of CD8+ T-cell responses in the peripheral blood and skin injection sites of melanoma patients treated with mRNA electroporated autologous dendritic cells (TriMixDC-MEL). Biomed Res Int. 2013;2013:976383. | |||||
| REF 30 | Structural Insights into How Protein Environments Tune the Spectroscopic Properties of a Noncanonical Amino Acid Fluorophore. Biochemistry. 2020 Sep 22;59(37):3401-3410. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

