Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T31479
(Former ID: TTDS00065)
|
|||||
| Target Name |
Phospholipase A2 (PLA2G1B)
|
|||||
| Synonyms |
Secreted phospholipase A(2); Phosphatidylcholine 2-acylhydrolase 1B; PLA2G1B; Group IB phospholipase A2
Click to Show/Hide
|
|||||
| Gene Name |
PLA2G1B
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 4 Target-related Diseases | + | ||||
| 1 | Leishmaniasis [ICD-11: 1F54] | |||||
| 2 | Peroxisomal disease [ICD-11: 5C57] | |||||
| 3 | Rosacea [ICD-11: ED90] | |||||
| 4 | Synthesis disorder [ICD-11: 5C52-5C59] | |||||
| Function |
PA2 catalyzes the calcium-dependent hydrolysis of the 2- acyl groups in 3-sn-phosphoglycerides, this releases glycerophospholipids and arachidonic acid that serve as the precursors of signal molecules.
Click to Show/Hide
|
|||||
| BioChemical Class |
Carboxylic ester hydrolase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.1.1.4
|
|||||
| Sequence |
MKLLVLAVLLTVAAADSGISPRAVWQFRKMIKCVIPGSDPFLEYNNYGCYCGLGGSGTPV
DELDKCCQTHDNCYDQAKKLDSCKFLLDNPYTHTYSYSCSGSAITCSSKNKECEAFICNC DRNAAICFSKAPYNKAHKNLDTKKYCQS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| ADReCS ID | BADD_A06627 | |||||
| HIT2.0 ID | T30TYH | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 5 Approved Drugs | + | ||||
| 1 | Cholic Acid | Drug Info | Approved | Synthesis disorder | [2] | |
| 2 | Clobetasol | Drug Info | Approved | Rosacea | [3] | |
| 3 | Clocortolone | Drug Info | Approved | Rosacea | [3] | |
| 4 | Diflorasone | Drug Info | Approved | Rosacea | [3] | |
| 5 | Miltefosine | Drug Info | Approved | Leishmaniasis | [4] | |
| Clinical Trial Drug(s) | [+] 6 Clinical Trial Drugs | + | ||||
| 1 | AllerB | Drug Info | Phase 2b | Allergy | [5] | |
| 2 | MANOALIDE | Drug Info | Phase 2 | Arthritis | [7] | |
| 3 | MRX-4 | Drug Info | Phase 2 | Allergic rhinitis | [8] | |
| 4 | MRX-6 | Drug Info | Phase 2 | Contact dermatitis | [9] | |
| 5 | URSOLIC ACID | Drug Info | Phase 2 | Metabolic syndrome x | [10] | |
| 6 | Rilapladib | Drug Info | Phase 1 | Cardiovascular disease | [11] | |
| Discontinued Drug(s) | [+] 8 Discontinued Drugs | + | ||||
| 1 | Darapladib | Drug Info | Discontinued in Phase 3 | Arteriosclerosis | [12], [13] | |
| 2 | BMY-30129 | Drug Info | Discontinued in Phase 2 | Pruritus | [14] | |
| 3 | EPC-K1 | Drug Info | Discontinued in Phase 2 | Nerve injury | [15] | |
| 4 | SC-106 | Drug Info | Discontinued in Phase 2 | Rheumatoid arthritis | [16] | |
| 5 | WAY-123641 | Drug Info | Discontinued in Phase 2 | Asthma | [17] | |
| 6 | PF-05212372 | Drug Info | Discontinued in Phase 1 | Asthma | [18] | |
| 7 | SB-435495 | Drug Info | Discontinued in Phase 1 | Arteriosclerosis | [19] | |
| 8 | YM-26734 | Drug Info | Terminated | Rheumatoid arthritis | [20] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Inhibitor | [+] 36 Inhibitor drugs | + | ||||
| 1 | Cholic Acid | Drug Info | [21] | |||
| 2 | Clobetasol | Drug Info | [1] | |||
| 3 | Diflorasone | Drug Info | [23] | |||
| 4 | Miltefosine | Drug Info | [24] | |||
| 5 | AllerB | Drug Info | [25] | |||
| 6 | MRX-4 | Drug Info | [28] | |||
| 7 | MRX-6 | Drug Info | [29] | |||
| 8 | URSOLIC ACID | Drug Info | [30] | |||
| 9 | BMY-30129 | Drug Info | [3], [34] | |||
| 10 | EPC-K1 | Drug Info | [3], [35] | |||
| 11 | SC-106 | Drug Info | [36] | |||
| 12 | WAY-123641 | Drug Info | [37] | |||
| 13 | PF-05212372 | Drug Info | [38] | |||
| 14 | SB-435495 | Drug Info | [39] | |||
| 15 | 2-Methyl-2,4-Pentanediol | Drug Info | [21] | |||
| 16 | 3,9-dihydroxy-2,10-diprenylpterocap-6a-ene | Drug Info | [42] | |||
| 17 | 4'-hydroxy-6,3',5'-triprenylisoflavonone | Drug Info | [42] | |||
| 18 | ABYSSINONE V | Drug Info | [42] | |||
| 19 | Acetate Ion | Drug Info | [21] | |||
| 20 | Alpha-D-Mannose | Drug Info | [21] | |||
| 21 | BM-162115 | Drug Info | [44] | |||
| 22 | BOLINAQUINONE | Drug Info | [45] | |||
| 23 | CACOSPONGIONOLIDE | Drug Info | [46] | |||
| 24 | CACOSPONGIONOLIDE B | Drug Info | [46] | |||
| 25 | Cacospongionolide E | Drug Info | [46] | |||
| 26 | HELENAQUINONE | Drug Info | [47] | |||
| 27 | Heptanoic Acid | Drug Info | [48] | |||
| 28 | Hexane-1,6-Diol | Drug Info | [21] | |||
| 29 | Lpc-Ether | Drug Info | [21] | |||
| 30 | LY178002 | Drug Info | [49] | |||
| 31 | Mepacrine | Drug Info | [50] | |||
| 32 | methylglyoxal | Drug Info | [21] | |||
| 33 | N-Tridecanoic Acid | Drug Info | [48] | |||
| 34 | P-Anisic Acid | Drug Info | [21] | |||
| 35 | Petrosaspongiolide M | Drug Info | [51] | |||
| 36 | Petrosaspongiolide P | Drug Info | [52] | |||
| Inducer | [+] 1 Inducer drugs | + | ||||
| 1 | Clocortolone | Drug Info | [22] | |||
| Modulator | [+] 7 Modulator drugs | + | ||||
| 1 | MANOALIDE | Drug Info | [26], [27] | |||
| 2 | Rilapladib | Drug Info | [31] | |||
| 3 | Darapladib | Drug Info | [32], [33] | |||
| 4 | YM-26734 | Drug Info | [40], [41] | |||
| 5 | AGN-190383 | Drug Info | [43] | |||
| 6 | SB-203347 | Drug Info | [53] | |||
| 7 | WA-8242-A1 | Drug Info | [54], [55] | |||
| Binder | [+] 1 Binder drugs | + | ||||
| 1 | LY256548 | Drug Info | [49] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Glycerophospholipid metabolism | hsa00564 | Affiliated Target |
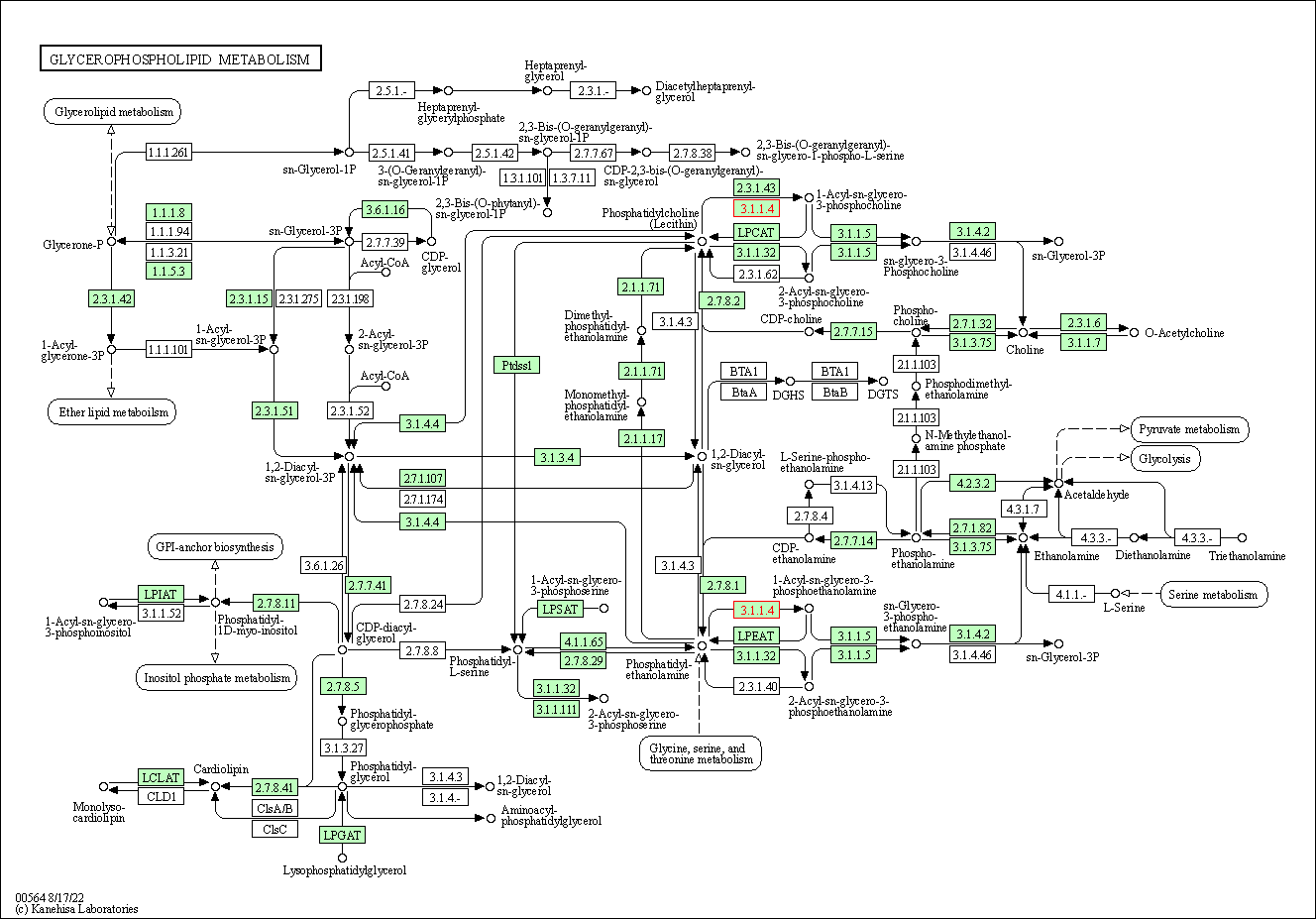
|
| Class: Metabolism => Lipid metabolism | Pathway Hierarchy | ||
| Ether lipid metabolism | hsa00565 | Affiliated Target |
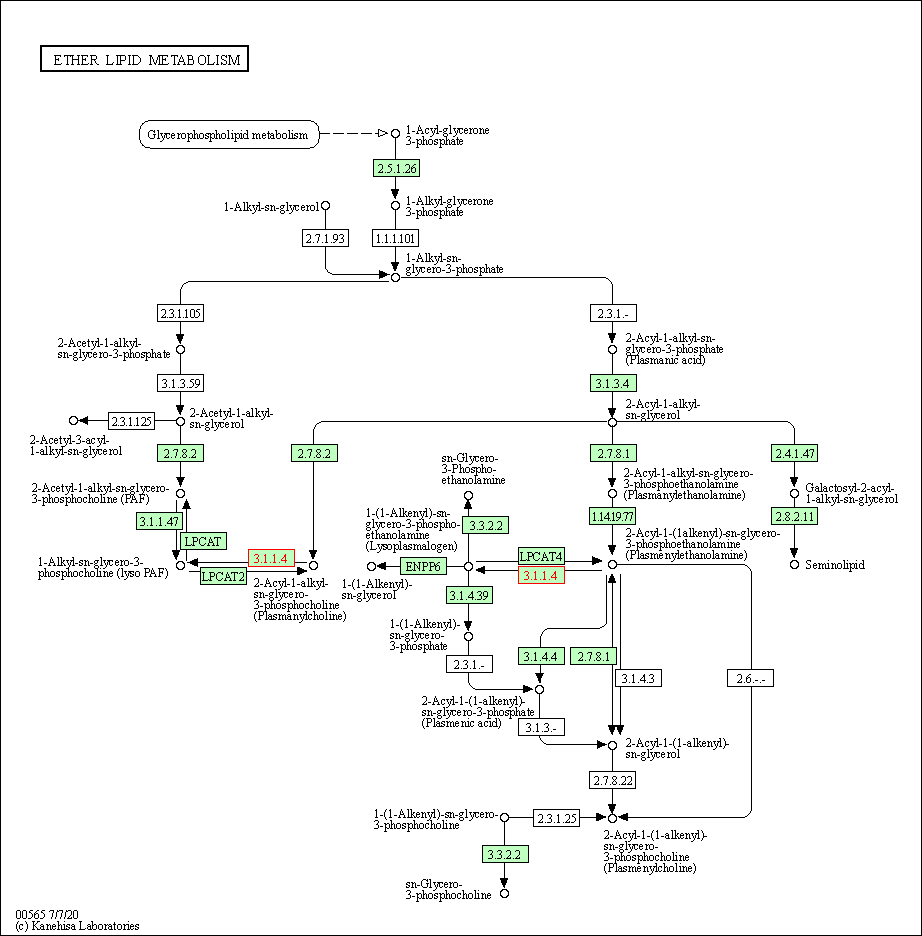
|
| Class: Metabolism => Lipid metabolism | Pathway Hierarchy | ||
| Arachidonic acid metabolism | hsa00590 | Affiliated Target |
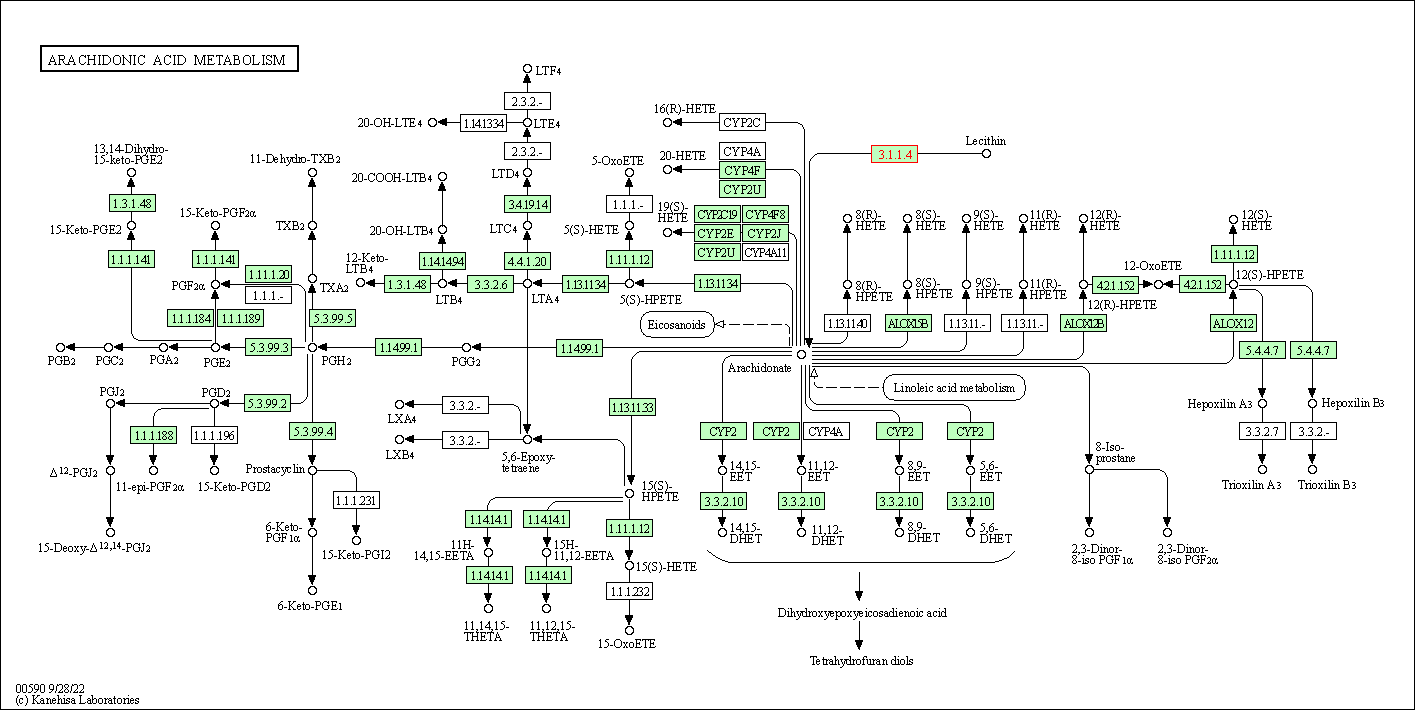
|
| Class: Metabolism => Lipid metabolism | Pathway Hierarchy | ||
| Linoleic acid metabolism | hsa00591 | Affiliated Target |
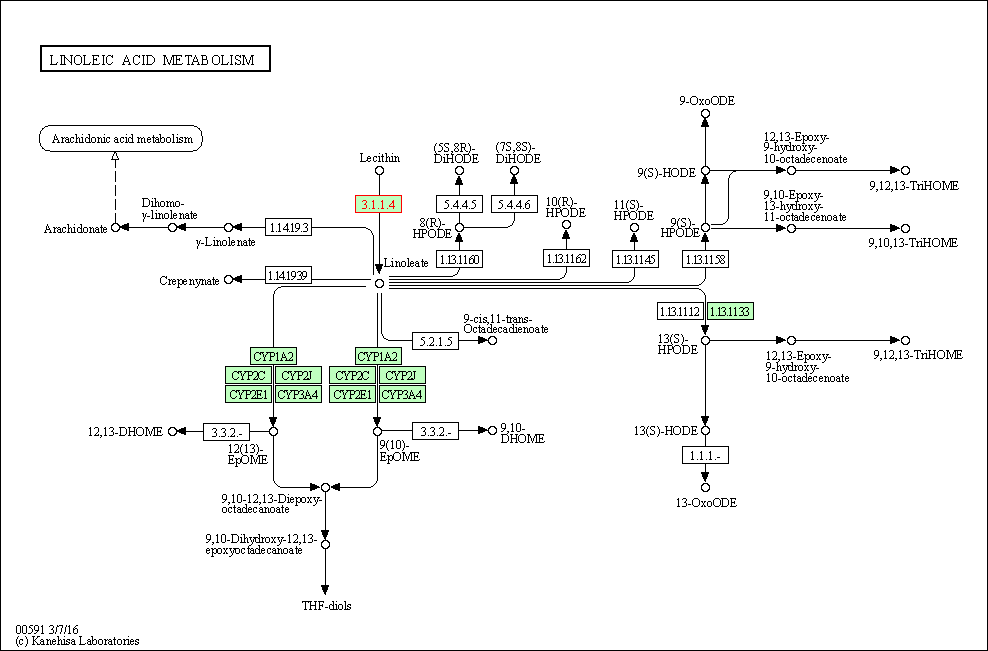
|
| Class: Metabolism => Lipid metabolism | Pathway Hierarchy | ||
| alpha-Linolenic acid metabolism | hsa00592 | Affiliated Target |
|
| Class: Metabolism => Lipid metabolism | Pathway Hierarchy | ||
| Ras signaling pathway | hsa04014 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Vascular smooth muscle contraction | hsa04270 | Affiliated Target |
|
| Class: Organismal Systems => Circulatory system | Pathway Hierarchy | ||
| Pancreatic secretion | hsa04972 | Affiliated Target |
|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Fat digestion and absorption | hsa04975 | Affiliated Target |
|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 1 | Degree centrality | 1.07E-04 | Betweenness centrality | 0.00E+00 |
|---|---|---|---|---|---|
| Closeness centrality | 1.44E-01 | Radiality | 1.17E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 3.00E+00 | Topological coefficient | 1.00E+00 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| BioCyc | [+] 1 BioCyc Pathways | + | ||||
| 1 | Phospholipases | |||||
| KEGG Pathway | [+] 10 KEGG Pathways | + | ||||
| 1 | Glycerophospholipid metabolism | |||||
| 2 | Ether lipid metabolism | |||||
| 3 | Arachidonic acid metabolism | |||||
| 4 | Linoleic acid metabolism | |||||
| 5 | alpha-Linolenic acid metabolism | |||||
| 6 | Metabolic pathways | |||||
| 7 | Ras signaling pathway | |||||
| 8 | Vascular smooth muscle contraction | |||||
| 9 | Pancreatic secretion | |||||
| 10 | Fat digestion and absorption | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | Fc-epsilon receptor I signaling in mast cells | |||||
| Reactome | [+] 3 Reactome Pathways | + | ||||
| 1 | Acyl chain remodelling of PC | |||||
| 2 | Acyl chain remodelling of PE | |||||
| 3 | Acyl chain remodelling of PI | |||||
| WikiPathways | [+] 1 WikiPathways | + | ||||
| 1 | Glycerophospholipid biosynthesis | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Utilization of epidermal phospholipase A2 inhibition to monitor topical steroid action. Br J Dermatol. 1984 Jul;111 Suppl 27:195-203. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 609). | |||||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 4 | 2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81. | |||||
| REF 5 | Clinical pipeline report, company report or official report of Anergis SA. | |||||
| REF 6 | ClinicalTrials.gov (NCT05717062) Randomized, Double-Blinded, Placebo-Controlled Study to Evaluate the Safety, Tolerability, and Efficacy of Intravenous Varespladib Followed by Oral Varespladib in Addition to Standard of Care in Subjects Bitten by Venomous Snakes. U.S.National Institutes of Health. | |||||
| REF 7 | In situ aquaculture methods for Dysidea avara (Demospongiae, Porifera) in the northwestern Mediterranean. Mar Drugs. 2010 May 26;8(6):1731-42. | |||||
| REF 8 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026713) | |||||
| REF 9 | ClinicalTrials.gov (NCT02031445) Double-Blind, Trial to Evaluate the Safety and Efficacy of MRX-6 Cream 2%. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT02337933) Effect of Ursolic Acid Administration on Insulin Sensitivity and Metabolic Syndrome. U.S. National Institutes of Health. | |||||
| REF 11 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018454) | |||||
| REF 12 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6696). | |||||
| REF 13 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800016591) | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005019) | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007770) | |||||
| REF 16 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007225) | |||||
| REF 17 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003918) | |||||
| REF 18 | ClinicalTrials.gov (NCT01415102) A First In Human Study In Healthy People To Evaluate Safety, Toleration And Time Course Of Plasma Concentration Of Single Inhaled Doses Of PF-05212372.. U.S. NationalInstitutes of Health. | |||||
| REF 19 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015443) | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005488) | |||||
| REF 21 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 22 | Clocortolone pivalate: a paired comparison clinical trial of a new topical steroid in eczema/atopic dermatitis. Cutis. 1980 Jan;25(1):96-8. | |||||
| REF 23 | Structures from powders: diflorasone diacetate. Steroids. 2009 Jan;74(1):102-11. | |||||
| REF 24 | Novel antifungal agents, targets or therapeutic strategies for the treatment of invasive fungal diseases: a review of the literature (2005-2009). Rev Iberoam Micol. 2009 Mar 31;26(1):15-22. | |||||
| REF 25 | Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998 Jun;101(6 Pt 1):747-54. | |||||
| REF 26 | Manoalide, a phospholipase A2 inhibitor, inhibits arachidonate incorporation and turnover in brain phospholipids of the awake rat. Neurochem Res. 1998 Oct;23(10):1251-7. | |||||
| REF 27 | The effects of the phospholipase A2 inhibitor, manoalide, on cartilage degradation, stromelysin expression, and synovial fluid cell count induced b... Arthritis Rheum. 1996 Aug;39(8):1292-9. | |||||
| REF 28 | Phospholipase A2, group IVA (cytosolic, calcium-dependent) (PLA2G4A). SciBX 1(41); doi:10.1038/scibx.2008.999. Nov. 13 2008 | |||||
| REF 29 | A novel treatment of contact dermatitis by topical application of phospholipase A2 inhibitor: a double-blind placebo-controlled pilot study. Int J Immunopathol Pharmacol. 2007 Jan-Mar;20(1):191-5. | |||||
| REF 30 | Synthesis of benzoyl phenyl benzoates as effective inhibitors for phospholipase A2 and hyaluronidase enzymes. Bioorg Med Chem Lett. 2005 Sep 15;15(18):4100-4. | |||||
| REF 31 | Effect of treatment for 12 weeks with rilapladib, a lipoprotein-associated phospholipase A2 inhibitor, on arterial inflammation as assessed with 18F-fluorodeoxyglucose-positron emission tomography imaging.J Am Coll Cardiol.2014 Jan 7-14;63(1):86-8. | |||||
| REF 32 | Darapladib, a reversible lipoprotein-associated phospholipase A2 inhibitor, for the oral treatment of atherosclerosis and coronary artery disease. IDrugs. 2009 Oct;12(10):648-55. | |||||
| REF 33 | Darapladib, a lipoprotein-associated phospholipase A2 inhibitor, in diabetic macular edema: a 3-month placebo-controlled study.Ophthalmology.2015 May;122(5):990-6. | |||||
| REF 34 | Inhibitor of phospholipase A2 blocks eicosanoid and platelet activating factor biosynthesis and has topical anti-inflammatory activity. J Pharmacol Exp Ther. 1994 Nov;271(2):852-9. | |||||
| REF 35 | Posttreatment with EPC-K1, an inhibitor of lipid peroxidation and of phospholipase A2 activity, reduces functional deficits after global ischemia in rats. Brain Res Bull. 1995;36(3):257-60. | |||||
| REF 36 | US patent application no. 6,673,908, Tumor necrosis factor receptor 2. | |||||
| REF 37 | Phosphodiesterase-IV inhibition, respiratory muscle relaxation and bronchodilation by WAY-PDA-641. J Pharmacol Exp Ther. 1994 Feb;268(2):888-96. | |||||
| REF 38 | Phagedena due to leishmaniasis. Immunologic and experimental studies. Ann Dermatol Syphiligr (Paris). 1976;103(1):23-30. | |||||
| REF 39 | The effect of lipoprotein-associated phospholipase A2 deficiency on pulmonary allergic responses in Aspergillus fumigatus sensitized mice. Respir Res. 2012 Nov 12;13:100. | |||||
| REF 40 | Simplified YM-26734 Inhibitors of Secreted Phospholipase A2 Group IIA | |||||
| REF 41 | Suppression of inflammatory responses to 12-O-tetradecanoyl-phorbol-13-acetate and carrageenin by YM-26734, a selective inhibitor of extracellular ... Br J Pharmacol. 1993 Sep;110(1):447-53. | |||||
| REF 42 | Phospholipase A2 Inhibitors from an Erythrina Species from Samoa J. Nat. Prod. 60(6):537-539 (1997). | |||||
| REF 43 | AGN 190383, a novel phospholipase inhibitor with topical anti-inflammatory activity. Agents Actions. 1991 Sep;34(1-2):70-2. | |||||
| REF 44 | Investigation on the effect of experimental phospholipase A2 inhibitors on the formyl-methionyl-leucyl-phenylalanine-stimulated chemotaxis of human leukocytes in vitro. Arzneimittelforschung. 1998 Jan;48(1):77-81. | |||||
| REF 45 | New sesquiterpene derivatives from the sponge Dysidea species with a selective inhibitor profile against human phospholipase A2 and other leukocyte... J Nat Prod. 2001 May;64(5):612-5. | |||||
| REF 46 | A new cacospongionolide inhibitor of human secretory phospholipase A2 from the Tyrrhenian sponge Fasciospongia cavernosa and absolute configuration... J Nat Prod. 1998 Jul;61(7):931-5. | |||||
| REF 47 | New bioactive halenaquinone derivatives from South Pacific marine sponges of the genus Xestospongia. Bioorg Med Chem. 2010 Aug 15;18(16):6006-11. | |||||
| REF 48 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 49 | The anti-inflammatory effects of LY178002 and LY256548. Agents Actions. 1989 Jun;27(3-4):300-2. | |||||
| REF 50 | Involvement of protein kinase C activation in L-leucine-induced stimulation of protein synthesis in l6 myotubes. Cytotechnology. 2003 Nov;43(1-3):97-103. | |||||
| REF 51 | Synthesis and pharmacological evaluation of a selected library of new potential anti-inflammatory agents bearing the gamma-hydroxybutenolide scaffo... J Med Chem. 2007 May 3;50(9):2176-84. | |||||
| REF 52 | Petrosaspongiolides M-R: new potent and selective phospholipase A2 inhibitors from the New Caledonian marine sponge Petrosaspongia nigra. J Nat Prod. 1998 May;61(5):571-5. | |||||
| REF 53 | SB 203347, an inhibitor of 14 kDa phospholipase A2, alters human neutrophil arachidonic acid release and metabolism and prolongs survival in murine... J Pharmacol Exp Ther. 1995 Sep;274(3):1254-62. | |||||
| REF 54 | WA8242A1, A2 and B, novel secretary phospholipase A2 inhibitors produced by Streptomyces violaceusniger. III. Structure elucidation and total synth... J Antibiot (Tokyo). 1998 Jul;51(7):655-64. | |||||
| REF 55 | WA8242A1, A2 and B, novel secretory phospholipase A2 inhibitors produced by Streptomyces violaceusniger. I. Taxonomy, production and purification. J Antibiot (Tokyo). 1998 Jan;51(1):1-7. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

