Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T31514
(Former ID: TTDC00023)
|
|||||
| Target Name |
Lysosomal alpha-glucosidase (GAA)
|
|||||
| Synonyms |
GAA; Aglucosidase alfa; Acid maltase
Click to Show/Hide
|
|||||
| Gene Name |
GAA
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Inborn carbohydrate metabolism error [ICD-11: 5C51] | |||||
| Function |
Essential for the degradation of glygogen to glucose in lysosomes.
Click to Show/Hide
|
|||||
| BioChemical Class |
Glycosylase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.2.1.20
|
|||||
| Sequence |
MGVRHPPCSHRLLAVCALVSLATAALLGHILLHDFLLVPRELSGSSPVLEETHPAHQQGA
SRPGPRDAQAHPGRPRAVPTQCDVPPNSRFDCAPDKAITQEQCEARGCCYIPAKQGLQGA QMGQPWCFFPPSYPSYKLENLSSSEMGYTATLTRTTPTFFPKDILTLRLDVMMETENRLH FTIKDPANRRYEVPLETPHVHSRAPSPLYSVEFSEEPFGVIVRRQLDGRVLLNTTVAPLF FADQFLQLSTSLPSQYITGLAEHLSPLMLSTSWTRITLWNRDLAPTPGANLYGSHPFYLA LEDGGSAHGVFLLNSNAMDVVLQPSPALSWRSTGGILDVYIFLGPEPKSVVQQYLDVVGY PFMPPYWGLGFHLCRWGYSSTAITRQVVENMTRAHFPLDVQWNDLDYMDSRRDFTFNKDG FRDFPAMVQELHQGGRRYMMIVDPAISSSGPAGSYRPYDEGLRRGVFITNETGQPLIGKV WPGSTAFPDFTNPTALAWWEDMVAEFHDQVPFDGMWIDMNEPSNFIRGSEDGCPNNELEN PPYVPGVVGGTLQAATICASSHQFLSTHYNLHNLYGLTEAIASHRALVKARGTRPFVISR STFAGHGRYAGHWTGDVWSSWEQLASSVPEILQFNLLGVPLVGADVCGFLGNTSEELCVR WTQLGAFYPFMRNHNSLLSLPQEPYSFSEPAQQAMRKALTLRYALLPHLYTLFHQAHVAG ETVARPLFLEFPKDSSTWTVDHQLLWGEALLITPVLQAGKAEVTGYFPLGTWYDLQTVPV EALGSLPPPPAAPREPAIHSEGQWVTLPAPLDTINVHLRAGYIIPLQGPGLTTTESRQQP MALAVALTKGGEARGELFWDDGESLEVLERGAYTQVIFLARNNTIVNELVRVTSEGAGLQ LQKVTVLGVATAPQQVLSNGVPVSNFTYSPDTKVLDICVSLLMGEQFLVSWC Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T12PIB | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 3 Clinical Trial Drugs | + | ||||
| 1 | BMN-701 | Drug Info | Phase 3 | Pompe disease | [3] | |
| 2 | Deoxynojirimycin | Drug Info | Phase 3 | Pompe disease | [4] | |
| 3 | GZ402666 | Drug Info | Phase 1 | Pompe disease | [7] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Modulator | [+] 2 Modulator drugs | + | ||||
| 1 | BMN-701 | Drug Info | [1], [7] | |||
| 2 | GZ402666 | Drug Info | [7] | |||
| Inhibitor | [+] 9 Inhibitor drugs | + | ||||
| 1 | Deoxynojirimycin | Drug Info | [9] | |||
| 2 | SALACINOL | Drug Info | [10] | |||
| 3 | (-)-uniflorine A | Drug Info | [11] | |||
| 4 | (R)-2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol | Drug Info | [12] | |||
| 5 | 7-O-b-D-Glucopyranosyl-a-homonojirimycin | Drug Info | [9] | |||
| 6 | Alpha-Homonojirimycin | Drug Info | [9] | |||
| 7 | N-adamantanemethyloxypentyl-1-deoxynojirimycin | Drug Info | [13] | |||
| 8 | UNIFLORINE B | Drug Info | [11] | |||
| 9 | VALIOLAMINE | Drug Info | [9] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Acetylcysteine | Ligand Info | |||||
| Structure Description | Crystal structure of human lysosomal acid-alpha-glucosidase, GAA, in complex with N-acetyl-cysteine | PDB:5NN4 | ||||
| Method | X-ray diffraction | Resolution | 1.83 Å | Mutation | No | [14] |
| PDB Sequence |
QCDVPPNSRF
90 DCAPDKAITQ100 EQCEARGCCY110 IPAKQGQPWC127 FFPPSYPSYK137 LENLSSSEMG 147 YTATLTRTTP157 TFFPKDILTL167 RLDVMMETEN177 RLHFTIKDPA187 NRRYEVPLET 197 APSPLYSVEF213 SEEPFGVIVH223 RQLDGRVLLN233 TTVAPLFFAD243 QFLQLSTSLP 253 SQYITGLAEH263 LSPLMLSTSW273 TRITLWNRDL283 APTPGANLYG293 SHPFYLALED 303 GGSAHGVFLL313 NSNAMDVVLQ323 PSPALSWRST333 GGILDVYIFL343 GPEPKSVVQQ 353 YLDVVGYPFM363 PPYWGLGFHL373 CRWGYSSTAI383 TRQVVENMTR393 AHFPLDVQWN 403 DLDYMDSRRD413 FTFNKDGFRD423 FPAMVQELHQ433 GGRRYMMIVD443 PAISSSGPAG 453 SYRPYDEGLR463 RGVFITNETG473 QPLIGKVWPG483 STAFPDFTNP493 TALAWWEDMV 503 AEFHDQVPFD513 GMWIDMNEPS523 NFIRGSEDGC533 PNNELENPPY543 VPGVVGGTLQ 553 AATICASSHQ563 FLSTHYNLHN573 LYGLTEAIAS583 HRALVKARGT593 RPFVISRSTF 603 AGHGRYAGHW613 TGDVWSSWEQ623 LASSVPEILQ633 FNLLGVPLVG643 ADVCGFLGNT 653 SEELCVRWTQ663 LGAFYPFMRN673 HNSLLSLPQE683 PYSFSEPAQQ693 AMRKALTLRY 703 ALLPHLYTLF713 HQAHVAGETV723 ARPLFLEFPK733 DSSTWTVDHQ743 LLWGEALLIT 753 PVLQAGKAEV763 TGYFPLGTWY773 DLQTVPIERE795 PAIHSEGQWV805 TLPAPLDTIN 815 VHLRAGYIIP825 LQGPGLTTTE835 SRQQPMALAV845 ALTKGGEARG855 ELFWDDGESL 865 EVLERGAYTQ875 VIFLARNNTI885 VNELVRVTSE895 GAGLQLQKVT905 VLGVATAPQQ 915 VLSNGVPVSN925 FTYSPDTKVL935 DICVSLLMGE945 QFLVSWC
|
|||||
|
|
||||||
| Ligand Name: Miglitol | Ligand Info | |||||
| Structure Description | Crystal structure of human lysosomal acid-alpha-glucosidase, GAA, in complex with N-hydroxyethyl-1-deoxynojirimycin | PDB:5NN6 | ||||
| Method | X-ray diffraction | Resolution | 2.00 Å | Mutation | No | [14] |
| PDB Sequence |
QCDVPPNSRF
90 DCAPDKAITQ100 EQCEARGCCY110 IPAKQGQPWC127 FFPPSYPSYK137 LENLSSSEMG 147 YTATLTRTTP157 TFFPKDILTL167 RLDVMMETEN177 RLHFTIKDPA187 NRRYEVPLAP 205 SPLYSVEFSE215 EPFGVIVHRQ225 LDGRVLLNTT235 VAPLFFADQF245 LQLSTSLPSQ 255 YITGLAEHLS265 PLMLSTSWTR275 ITLWNRDLAP285 TPGANLYGSH295 PFYLALEDGG 305 SAHGVFLLNS315 NAMDVVLQPS325 PALSWRSTGG335 ILDVYIFLGP345 EPKSVVQQYL 355 DVVGYPFMPP365 YWGLGFHLCR375 WGYSSTAITR385 QVVENMTRAH395 FPLDVQWNDL 405 DYMDSRRDFT415 FNKDGFRDFP425 AMVQELHQGG435 RRYMMIVDPA445 ISSSGPAGSY 455 RPYDEGLRRG465 VFITNETGQP475 LIGKVWPGST485 AFPDFTNPTA495 LAWWEDMVAE 505 FHDQVPFDGM515 WIDMNEPSNF525 IRGSEDGCPN535 NELENPPYVP545 GVVGGTLQAA 555 TICASSHQFL565 STHYNLHNLY575 GLTEAIASHR585 ALVKARGTRP595 FVISRSTFAG 605 HGRYAGHWTG615 DVWSSWEQLA625 SSVPEILQFN635 LLGVPLVGAD645 VCGFLGNTSE 655 ELCVRWTQLG665 AFYPFMRNHN675 SLLSLPQEPY685 SFSEPAQQAM695 RKALTLRYAL 705 LPHLYTLFHQ715 AHVAGETVAR725 PLFLEFPKDS735 STWTVDHQLL745 WGEALLITPV 755 LQAGKAEVTG765 YFPLGTWYDL775 QTVPIEREPA797 IHSEGQWVTL807 PAPLDTINVH 817 LRAGYIIPLQ827 GPGLTTTESR837 QQPMALAVAL847 TKGGEARGEL857 FWDDGESLEV 867 LERGAYTQVI877 FLARNNTIVN887 ELVRVTSEGA897 GLQLQKVTVL907 GVATAPQQVL 917 SNGVPVSNFT927 YSPDTKVLDI937 VSLLMGEQFL948 VSWC
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Galactose metabolism | hsa00052 | Affiliated Target |
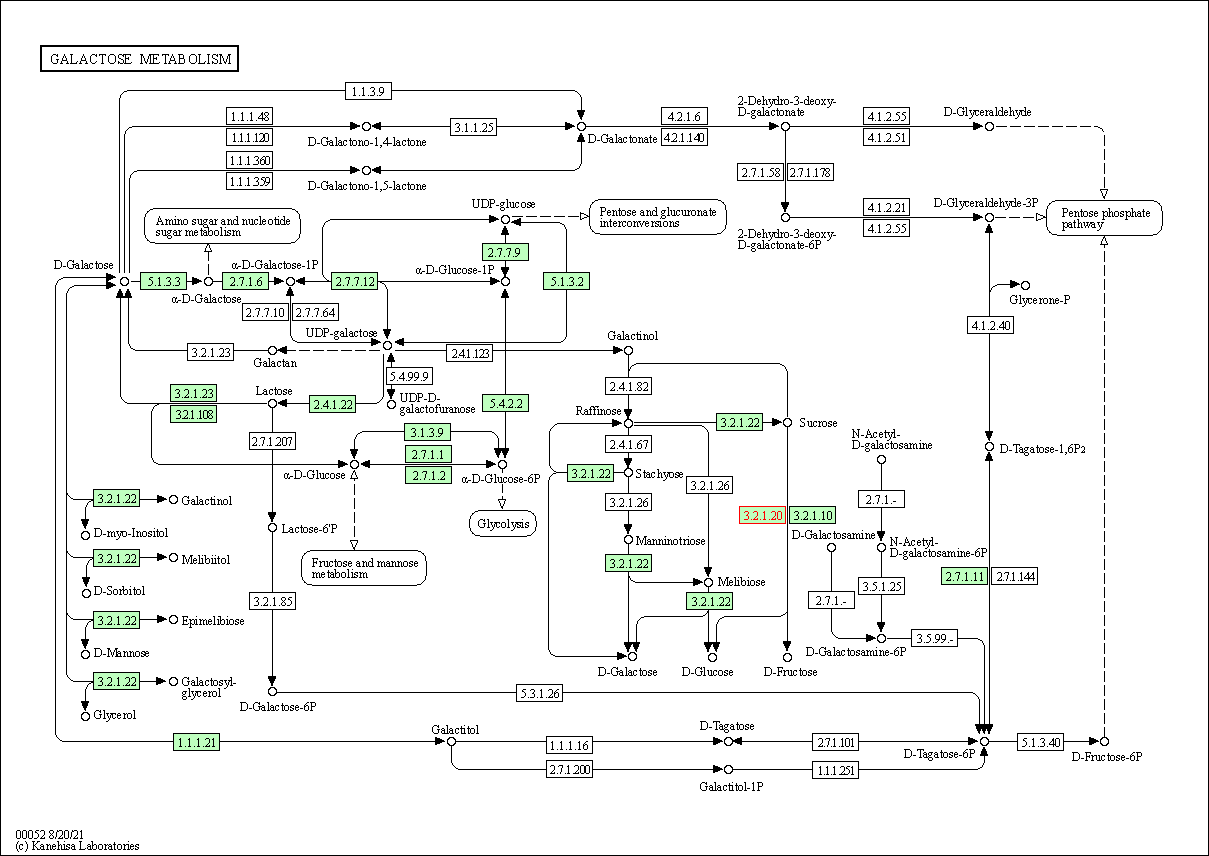
|
| Class: Metabolism => Carbohydrate metabolism | Pathway Hierarchy | ||
| Starch and sucrose metabolism | hsa00500 | Affiliated Target |
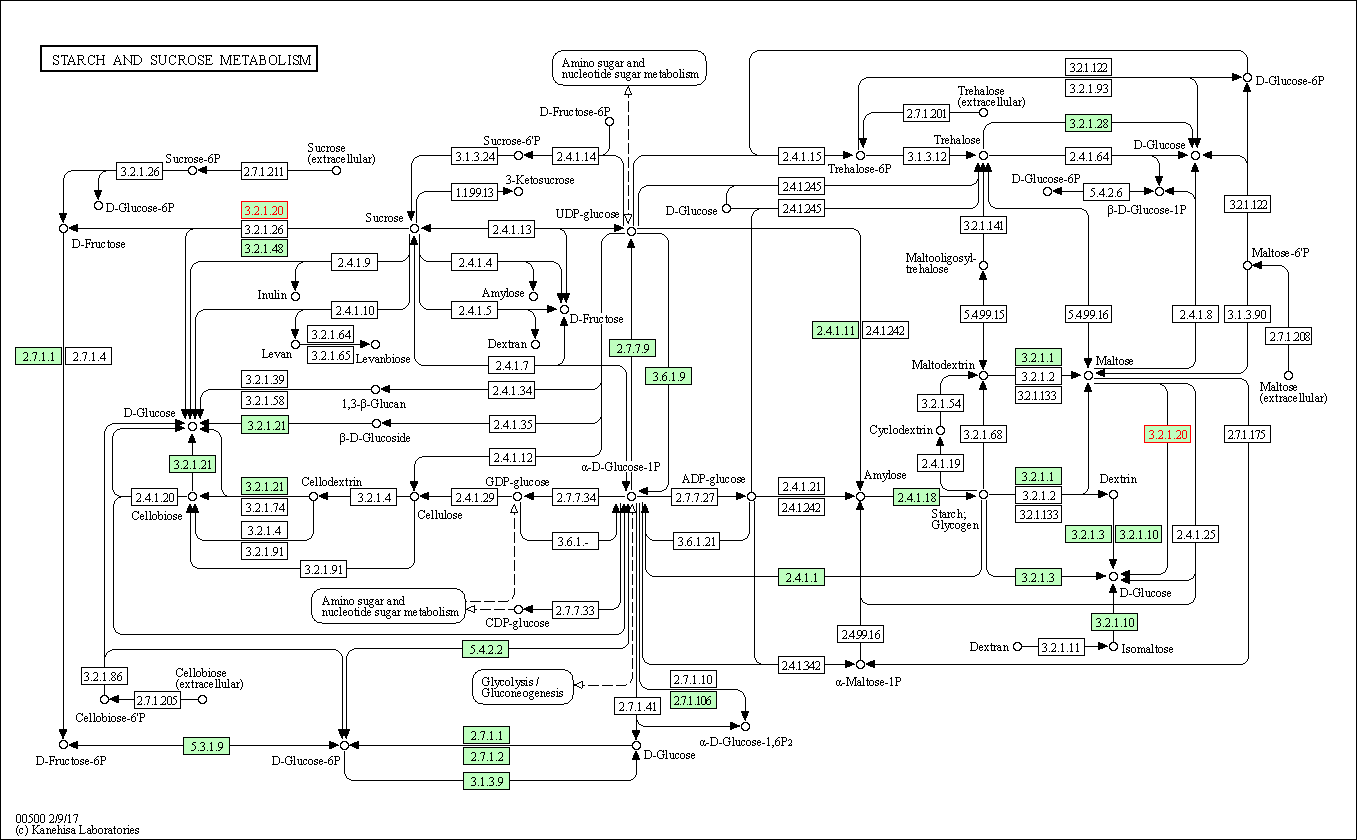
|
| Class: Metabolism => Carbohydrate metabolism | Pathway Hierarchy | ||
| Lysosome | hsa04142 | Affiliated Target |
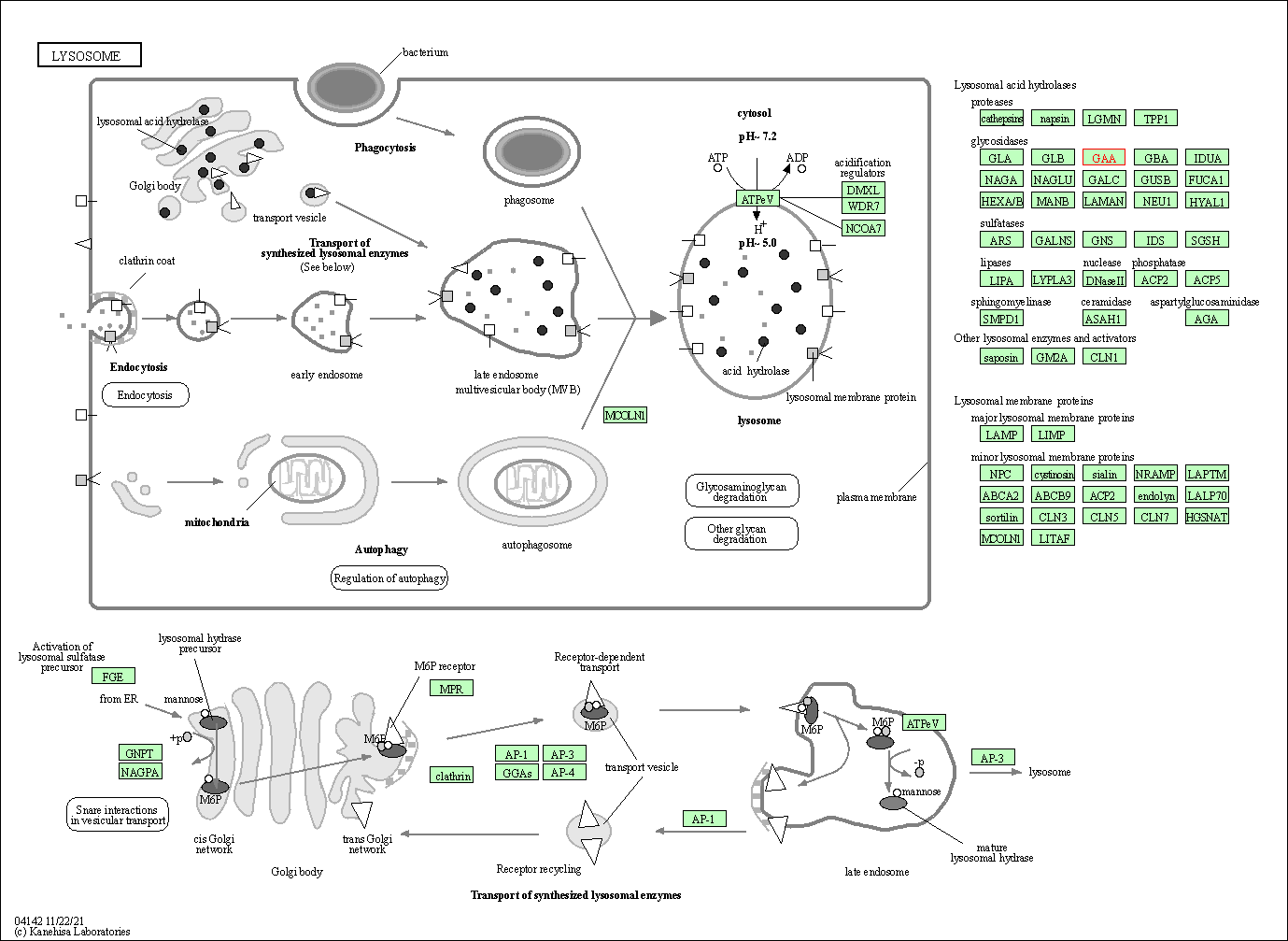
|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
| Degree | 1 | Degree centrality | 1.07E-04 | Betweenness centrality | 0.00E+00 |
|---|---|---|---|---|---|
| Closeness centrality | 1.21E-01 | Radiality | 1.05E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 8.00E+00 | Topological coefficient | 1.00E+00 | Eccentricity | 14 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 4 KEGG Pathways | + | ||||
| 1 | Galactose metabolism | |||||
| 2 | Starch and sucrose metabolism | |||||
| 3 | Metabolic pathways | |||||
| 4 | Lysosome | |||||
| Pathwhiz Pathway | [+] 1 Pathwhiz Pathways | + | ||||
| 1 | Galactose Metabolism | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | Notch-mediated HES/HEY network | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of BioMarin Pharma. | |||||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2021. Application Number: 761194. | |||||
| REF 3 | ClinicalTrials.gov (NCT01924845) BMN 701 Phase 3 in rhGAA Exposed Subjects With Late Onset Pompe Disease (INSPIRE Study). U.S. National Institutes of Health. | |||||
| REF 4 | The pharmacological chaperone 1-deoxynojirimycin increases the activity and lysosomal trafficking of multiple mutant forms of acid alpha-glucosidase. Hum Mutat. 2009 Dec;30(12):1683-92. | |||||
| REF 5 | ClinicalTrials.gov (NCT04174105) A Phase 1/2, Open-Label, Ascending-Dose Clinical Study to Evaluate the Safety and Preliminary Efficacy of AT845, an AAV8-Delivered Gene Transfer Therapy in Patients With Late Onset Pompe Disease. U.S.National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT04093349) Phase 1/2, Dose-escalation Study to Evaluate the Safety, Tolerability and Efficacy of a Single Intravenous Infusion of SPK-3006 in Adults With Late-onset Pompe Disease. U.S.National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT01898364) Safety and Efficacy Evaluation of Repeat neoGAA Dosing in Late Onset Pompe Disease Patients.. U.S. National Institutes of Health. | |||||
| REF 8 | Discovery of a novel noniminosugar acid alpha glucosidase chaperone series. J Med Chem. 2012 Sep 13;55(17):7546-59. | |||||
| REF 9 | In vitro inhibition of glycogen-degrading enzymes and glycosidases by six-membered sugar mimics and their evaluation in cell cultures. Bioorg Med Chem. 2008 Aug 1;16(15):7330-6. | |||||
| REF 10 | Alpha-glucosidase inhibitor from Kothala-himbutu (Salacia reticulata WIGHT). J Nat Prod. 2008 Jun;71(6):981-4. | |||||
| REF 11 | Total synthesis of (-)-uniflorine A. J Nat Prod. 2009 Nov;72(11):2058-60. | |||||
| REF 12 | Homonojirimycin isomers and N-alkylated homonojirimycins: structural and conformational basis of inhibition of glycosidases. J Med Chem. 1998 Jul 2;41(14):2565-71. | |||||
| REF 13 | Synthesis and evaluation of D-gluco-pyranocyclopropyl amines as potential glucosidase inhibitors. Bioorg Med Chem Lett. 2009 Dec 1;19(23):6600-3. | |||||
| REF 14 | Structure of human lysosomal acid Alpha-glucosidase-a guide for the treatment of Pompe disease. Nat Commun. 2017 Oct 24;8(1):1111. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

