Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T46482
(Former ID: TTDS00311)
|
|||||
| Target Name |
Toll-like receptor 7 (TLR7)
|
|||||
| Synonyms |
Toll-like receptor 7
Click to Show/Hide
|
|||||
| Gene Name |
TLR7
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Malaria [ICD-11: 1F40-1F45] | |||||
| 2 | Skin cancer [ICD-11: 2C30-2C37] | |||||
| Function |
Key component of innate and adaptive immunity. TLRs (Toll-like receptors) control host immune response against pathogens through recognition of molecular patterns specific to microorganisms. TLR7 is a nucleotide-sensing TLR which is activated by single-stranded RNA. Acts via MYD88 and TRAF6, leading to NF-kappa-B activation, cytokine secretion and the inflammatory response (By similarity).
Click to Show/Hide
|
|||||
| BioChemical Class |
Toll-like receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MVFPMWTLKRQILILFNIILISKLLGARWFPKTLPCDVTLDVPKNHVIVDCTDKHLTEIP
GGIPTNTTNLTLTINHIPDISPASFHRLDHLVEIDFRCNCVPIPLGSKNNMCIKRLQIKP RSFSGLTYLKSLYLDGNQLLEIPQGLPPSLQLLSLEANNIFSIRKENLTELANIEILYLG QNCYYRNPCYVSYSIEKDAFLNLTKLKVLSLKDNNVTAVPTVLPSTLTELYLYNNMIAKI QEDDFNNLNQLQILDLSGNCPRCYNAPFPCAPCKNNSPLQIPVNAFDALTELKVLRLHSN SLQHVPPRWFKNINKLQELDLSQNFLAKEIGDAKFLHFLPSLIQLDLSFNFELQVYRASM NLSQAFSSLKSLKILRIRGYVFKELKSFNLSPLHNLQNLEVLDLGTNFIKIANLSMFKQF KRLKVIDLSVNKISPSGDSSEVGFCSNARTSVESYEPQVLEQLHYFRYDKYARSCRFKNK EASFMSVNESCYKYGQTLDLSKNSIFFVKSSDFQHLSFLKCLNLSGNLISQTLNGSEFQP LAELRYLDFSNNRLDLLHSTAFEELHKLEVLDISSNSHYFQSEGITHMLNFTKNLKVLQK LMMNDNDISSSTSRTMESESLRTLEFRGNHLDVLWREGDNRYLQLFKNLLKLEELDISKN SLSFLPSGVFDGMPPNLKNLSLAKNGLKSFSWKKLQCLKNLETLDLSHNQLTTVPERLSN CSRSLKNLILKNNQIRSLTKYFLQDAFQLRYLDLSSNKIQMIQKTSFPENVLNNLKMLLL HHNRFLCTCDAVWFVWWVNHTEVTIPYLATDVTCVGPGAHKGQSVISLDLYTCELDLTNL ILFSLSISVSLFLMVMMTASHLYFWDVWYIYHFCKAKIKGYQRLISPDCCYDAFIVYDTK DPAVTEWVLAELVAKLEDPREKHFNLCLEERDWLPGQPVLENLSQSIQLSKKTVFVMTDK YAKTENFKIAFYLSHQRLMDEKVDVIILIFLEKPFQKSKFLQLRKRLCGSSVLEWPTNPQ AHPYFWQCLKNALATDNHVAYSQVFKETV Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T92RZK | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Imiquimod | Drug Info | Approved | Skin cancer | [4], [5] | |
| Clinical Trial Drug(s) | [+] 12 Clinical Trial Drugs | + | ||||
| 1 | ANA773 | Drug Info | Phase 2a | Hepatitis C virus infection | [6] | |
| 2 | AZD-8848 | Drug Info | Phase 2 | Allergic rhinitis | [8] | |
| 3 | GS-9620 | Drug Info | Phase 2 | Hepatitis B virus infection | [9] | |
| 4 | GSK2245035 | Drug Info | Phase 2 | Asthma | [10] | |
| 5 | IMO-3100 | Drug Info | Phase 2 | Psoriasis vulgaris | [11] | |
| 6 | Isatoribine | Drug Info | Phase 2 | Hepatitis C virus infection | [12] | |
| 7 | LOXORIBINE | Drug Info | Phase 2 | Immune System disease | [13], [14] | |
| 8 | PF-4878691 | Drug Info | Phase 2 | Melanoma | [15] | |
| 9 | Resiquimod | Drug Info | Phase 2 | Actinic keratosis | [16] | |
| 10 | CPG 52364 | Drug Info | Phase 1 | Systemic lupus erythematosus | [23] | |
| 11 | DV-1179 | Drug Info | Phase 1 | Autoimmune disease | [24] | |
| 12 | MEDI9197 | Drug Info | Phase 1 | Solid tumour/cancer | [25] | |
| Discontinued Drug(s) | [+] 3 Discontinued Drugs | + | ||||
| 1 | IM0-8400 | Drug Info | Discontinued in Phase 1/2 | Diffuse large B-cell lymphoma | [26] | |
| 2 | ANA-975 | Drug Info | Discontinued in Phase 1 | Immune System disease | [27] | |
| 3 | IPH-3201 | Drug Info | Terminated | Solid tumour/cancer | [28] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Agonist | [+] 10 Agonist drugs | + | ||||
| 1 | Imiquimod | Drug Info | [1] | |||
| 2 | ANA773 | Drug Info | [6] | |||
| 3 | AZD-8848 | Drug Info | [29] | |||
| 4 | Isatoribine | Drug Info | [32] | |||
| 5 | LOXORIBINE | Drug Info | [33], [34] | |||
| 6 | PF-4878691 | Drug Info | [35] | |||
| 7 | MEDI9197 | Drug Info | [25] | |||
| 8 | ANA-975 | Drug Info | [32] | |||
| 9 | TMX-202 | Drug Info | [40] | |||
| 10 | TMX-30X | Drug Info | [40] | |||
| Modulator | [+] 6 Modulator drugs | + | ||||
| 1 | GS-9620 | Drug Info | [9] | |||
| 2 | GSK2245035 | Drug Info | [30] | |||
| 3 | IMO-3100 | Drug Info | [31] | |||
| 4 | Resiquimod | Drug Info | [36] | |||
| 5 | DV-1179 | Drug Info | [38] | |||
| 6 | IPH-3201 | Drug Info | [40] | |||
| Antagonist | [+] 2 Antagonist drugs | + | ||||
| 1 | CPG 52364 | Drug Info | [37] | |||
| 2 | IM0-8400 | Drug Info | [39] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Neutrophil extracellular trap formation | hsa04613 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Toll-like receptor signaling pathway | hsa04620 | Affiliated Target |
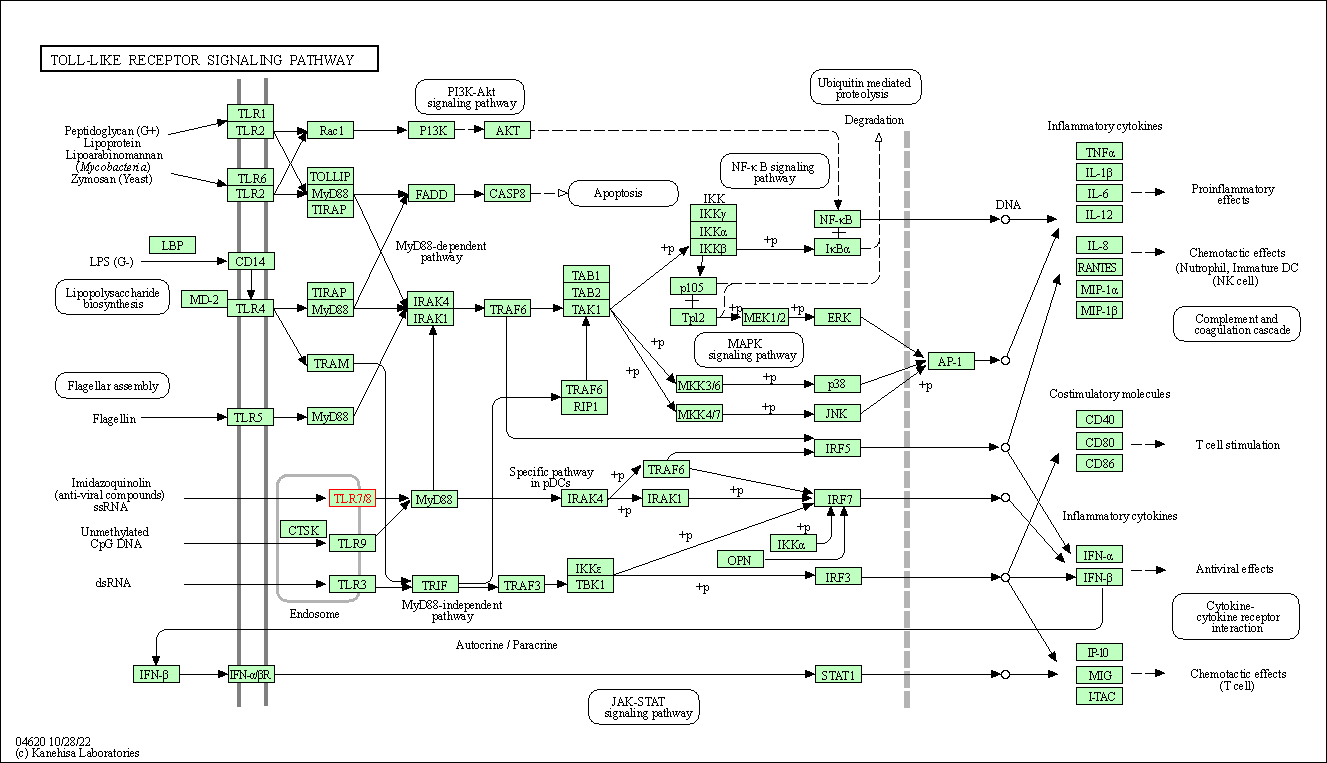
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 5 | Degree centrality | 5.37E-04 | Betweenness centrality | 1.70E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.14E-01 | Radiality | 1.38E+01 | Clustering coefficient | 3.00E-01 |
| Neighborhood connectivity | 3.68E+01 | Topological coefficient | 3.17E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 3 KEGG Pathways | + | ||||
| 1 | Toll-like receptor signaling pathway | |||||
| 2 | Measles | |||||
| 3 | Influenza A | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | TCR Signaling Pathway | |||||
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | Toll receptor signaling pathway | |||||
| Reactome | [+] 4 Reactome Pathways | + | ||||
| 1 | Trafficking and processing of endosomal TLR | |||||
| 2 | TRAF6 mediated IRF7 activation in TLR7/8 or 9 signaling | |||||
| 3 | TRAF6 mediated induction of NFkB and MAP kinases upon TLR7/8 or 9 activation | |||||
| 4 | MyD88 dependent cascade initiated on endosome | |||||
| WikiPathways | [+] 5 WikiPathways | + | ||||
| 1 | Toll-like receptor signaling pathway | |||||
| 2 | Toll-Like Receptors Cascades | |||||
| 3 | MyD88 dependent cascade initiated on endosome | |||||
| 4 | Trafficking and processing of endosomal TLR | |||||
| 5 | Regulation of toll-like receptor signaling pathway | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin. J Invest Dermatol. 2009 Nov;129(11):2676-85. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7198). | |||||
| REF 3 | Emerging drugs for rheumatoid arthritis. Expert Opin Emerg Drugs. 2008 Mar;13(1):175-96. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5003). | |||||
| REF 5 | New drugs in development for the treatment of endometriosis. Expert Opin Investig Drugs. 2008 Aug;17(8):1187-202. | |||||
| REF 6 | Clinical pipeline report, company report or official report of Anadys Pharmaceuticals (2011). | |||||
| REF 7 | ClinicalTrials.gov (NCT04895696) A Phase 2, Multicenter, Randomized, Double-blind, Placebo-Controlled, Study to Evaluate the Efficacy and Safety of Afimetoran in Participants With Active Systemic Lupus Erythematosus. U.S.National Institutes of Health. | |||||
| REF 8 | ClinicalTrials.gov (NCT01185080) Efficacy Study in Allergic Rhinitis Patients After Intranasal Administration of AZD8848. U.S. National Institutes of Health. | |||||
| REF 9 | Pharmacokinetic and pharmacodynamic properties of GS-9620, a novel Toll-like receptor 7 agonist, demonstrate interferon-stimulated gene induction without detectable serum interferon at low oral doses. J Pharmacol Exp Ther. 2014 Jan;348(1):96-105. | |||||
| REF 10 | ClinicalTrials.gov (NCT01607372) A Study to Investigate the Safety, Pharmacodynamics and Efficacy Against Allergic Reactivity of Repeat Intranasal Administration of the TLR7 Agonist GSK2245035 in Subjects With Respiratory Allergies. U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT01622348) Trial of IMO-3100 in Patients With Moderate to Severe Plaque Psoriasis. U.S. National Institutes of Health. | |||||
| REF 12 | ClinicalTrials.gov (NCT00480831) A Study of PRO95780 in Patients With Previously Untreated, Advanced-Stage Non-Small Cell Lung Cancer (APM4074g). U.S. National Institutes of Health. | |||||
| REF 13 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5018). | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002417) | |||||
| REF 15 | ClinicalTrials.gov (NCT00189332) Use of 852A in Metastatic Cutaneous Melanoma.. U.S. National Institutes of Health. | |||||
| REF 16 | ClinicalTrials.gov (NCT01583816) Dose-finding, Safety, Efficacy Trial of Topical Resiquimod in Patients With Multiple Actinic Keratosis Lesions. U.S. National Institutes of Health. | |||||
| REF 17 | ClinicalTrials.gov (NCT04101357) Safety, Pharmacokinetics, Pharmacodynamics, and Preliminary Efficacy Trial of BNT411. U.S. National Institutes of Health. | |||||
| REF 18 | ClinicalTrials.gov (NCT03416335) A Study of DSP-0509 in Patients With Advanced Solid Tumors to Determine the Safety and the Pharmacokinetic Profile. U.S. National Institutes of Health. | |||||
| REF 19 | ClinicalTrials.gov (NCT03435640) A Study of NKTR-262 in Combination With Bempegaldesleukin (NKTR-214) and With Bempegaldesleukin Plus Nivolumab in Patients With Locally Advanced or Metastatic Solid Tumor Malignancies (REVEAL). U.S. National Institutes of Health. | |||||
| REF 20 | Clinical pipeline report, company report or official report of Roche. | |||||
| REF 21 | ClinicalTrials.gov (NCT03301896) Study of the Safety and Efficacy of LHC165 Single Agent and in Combination With PDR001 in Patients With Advanced Malignancies. U.S. National Institutes of Health. | |||||
| REF 22 | ClinicalTrials.gov (NCT03291002) Study of Intratumoral CV8102 in cMEL, cSCC, hnSCC, and ACC. U.S. National Institutes of Health. | |||||
| REF 23 | Treatment of systemic lupus erythematosus: new therapeutic avenues and blind alleys. Nat Rev Rheumatol. 2014 Jan;10(1):23-34. | |||||
| REF 24 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 25 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 26 | Clinical pipeline report, company report or official report of Idera Pharmaceuticals. | |||||
| REF 27 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020825) | |||||
| REF 28 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026574) | |||||
| REF 29 | Repeated intranasal TLR7 stimulation reduces allergen responsiveness in allergic rhinitis. Respir Res. 2012 Jun 22;13:53. | |||||
| REF 30 | Early clinical evaluation of the intranasal TLR7 agonist GSK2245035: Use of translational biomarkers to guide dosing and confirm target engagement. Clin Pharmacol Ther. 2015 Oct;98(4):369-80. | |||||
| REF 31 | Company report (Iderapharma) | |||||
| REF 32 | Discovery of ANA975: an oral prodrug of the TLR-7 agonist isatoribine. Nucleosides Nucleotides Nucleic Acids. 2007;26(6-7):635-40. | |||||
| REF 33 | The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol. 2003 Nov;33(11):2987-97. | |||||
| REF 34 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 35 | The innate immune response, clinical outcomes, and ex vivo HCV antiviral efficacy of a TLR7 agonist (PF-4878691). Clin Pharmacol Ther. 2011 Jun;89(6):821-9. | |||||
| REF 36 | TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848).Vaccine.2005 Nov 1;23(45):5263-70. | |||||
| REF 37 | Coley Pharmaceutical Group Diversifies Pipeline with First-in-Class TLR Antagonist for the Treatment of Systemic Lupus Erythematosus. Lupus Foundation of America, Inc. 2007. | |||||
| REF 38 | Company report (Dynavax) | |||||
| REF 39 | Clinical pipeline report, company report or official report of Idera Pharmaceuticals. | |||||
| REF 40 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1757). | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

