Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T61622
(Former ID: TTDS00451)
|
|||||
| Target Name |
Angiotensinogenase renin (REN)
|
|||||
| Synonyms |
Renin; Angiotensinogenase
Click to Show/Hide
|
|||||
| Gene Name |
REN
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Hypertension [ICD-11: BA00-BA04] | |||||
| Function |
Renin is a highly specific endopeptidase, whose only knownfunction is to generate angiotensin I from angiotensinogen in the plasma, initiating a cascade of reactions that produce an elevation of blood pressure and increased sodium retention by the kidney.
Click to Show/Hide
|
|||||
| BioChemical Class |
Peptidase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.4.23.15
|
|||||
| Sequence |
MDGWRRMPRWGLLLLLWGSCTFGLPTDTTTFKRIFLKRMPSIRESLKERGVDMARLGPEW
SQPMKRLTLGNTTSSVILTNYMDTQYYGEIGIGTPPQTFKVVFDTGSSNVWVPSSKCSRL YTACVYHKLFDASDSSSYKHNGTELTLRYSTGTVSGFLSQDIITVGGITVTQMFGEVTEM PALPFMLAEFDGVVGMGFIEQAIGRVTPIFDNIISQGVLKEDVFSFYYNRDSENSQSLGG QIVLGGSDPQHYEGNFHYINLIKTGVWQIQMKGVSVGSSTLLCEDGCLALVDTGASYISG STSSIEKLMEALGAKKRLFDYVVKCNEGPTLPDISFHLGGKEYTLTSADYVFQESYSSKK LCTLAIHAMDIPPPTGPTWALGATFIRKFYTEFDRRNNRIGFALAR Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| ADReCS ID | BADD_A02216 ; BADD_A05470 | |||||
| HIT2.0 ID | T82P0Z | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 2 Approved Drugs | + | ||||
| 1 | Aliskiren | Drug Info | Approved | Hypertension | [2], [3], [4] | |
| 2 | Remikiren | Drug Info | Approved | Hypertension | [5] | |
| Clinical Trial Drug(s) | [+] 5 Clinical Trial Drugs | + | ||||
| 1 | SPP-600 | Drug Info | Phase 2 | Hypertension | [8] | |
| 2 | SR-43845 | Drug Info | Phase 2 | Glaucoma/ocular hypertension | [9] | |
| 3 | TAK-272 | Drug Info | Phase 2 | Type-2 diabetes | [10] | |
| 4 | ACT-178882 | Drug Info | Phase 1 | Cardiovascular disease | [12] | |
| 5 | VTP-27999 | Drug Info | Phase 1 | Hypertension | [13] | |
| Discontinued Drug(s) | [+] 13 Discontinued Drugs | + | ||||
| 1 | Enalkiren | Drug Info | Discontinued in Phase 2 | Glaucoma/ocular hypertension | [14] | |
| 2 | FK-906 | Drug Info | Discontinued in Phase 2 | Hypertension | [15] | |
| 3 | ZANKIREN | Drug Info | Discontinued in Phase 2 | Hypertension | [16] | |
| 4 | SPP-1148 | Drug Info | Discontinued in Phase 1 | Hypertension | [18] | |
| 5 | SPP-676 | Drug Info | Discontinued in Phase 1 | Hypertension | [19] | |
| 6 | A-74273 | Drug Info | Terminated | Hypertension | [21] | |
| 7 | BILA-2157BS | Drug Info | Terminated | Hypertension | [22] | |
| 8 | Ciprokiren | Drug Info | Terminated | Hypertension | [23] | |
| 9 | ES-1005 | Drug Info | Terminated | Hypertension | [24] | |
| 10 | JTP-2724 | Drug Info | Terminated | Hypertension | [25] | |
| 11 | JTP-4761 | Drug Info | Terminated | Hypertension | [26] | |
| 12 | SC-56525 | Drug Info | Terminated | Hypertension | [27] | |
| 13 | SQ-33800 | Drug Info | Terminated | Hypertension | [28] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 43 Inhibitor drugs | + | ||||
| 1 | Aliskiren | Drug Info | [1] | |||
| 2 | Remikiren | Drug Info | [5] | |||
| 3 | SPP-600 | Drug Info | [29] | |||
| 4 | SR-43845 | Drug Info | [30], [31] | |||
| 5 | ACT-178882 | Drug Info | [33] | |||
| 6 | VTP-27999 | Drug Info | [34] | |||
| 7 | Enalkiren | Drug Info | [35] | |||
| 8 | FK-906 | Drug Info | [36] | |||
| 9 | ZANKIREN | Drug Info | [37] | |||
| 10 | SPP-1148 | Drug Info | [34] | |||
| 11 | SPP-676 | Drug Info | [34] | |||
| 12 | A-74273 | Drug Info | [38] | |||
| 13 | BILA-2157BS | Drug Info | [39] | |||
| 14 | Ciprokiren | Drug Info | [40] | |||
| 15 | ES-1005 | Drug Info | [41] | |||
| 16 | JTP-2724 | Drug Info | [42] | |||
| 17 | JTP-4761 | Drug Info | [43] | |||
| 18 | SC-56525 | Drug Info | [44] | |||
| 19 | SQ-33800 | Drug Info | [45] | |||
| 20 | (H-261)Boc-His-Pro-Phe-His-Leu(OH)-Val-Ile-His-OH | Drug Info | [46] | |||
| 21 | 1-Hydroxy-2-Amino-3-Cyclohexylpropane | Drug Info | [47] | |||
| 22 | 1-Methyl-2-Oxy-5,5-Dimethyl Pyrrolidine | Drug Info | [47] | |||
| 23 | 2-Cyclopropylmethylenepropanal | Drug Info | [47] | |||
| 24 | 2-Methyl-3-(2-Aminothiazolo)Propanal | Drug Info | [48] | |||
| 25 | 3-Phenyl-1,2-Propandiol | Drug Info | [47] | |||
| 26 | CP-305202 | Drug Info | [49] | |||
| 27 | Dimethylformamide | Drug Info | [50] | |||
| 28 | ES-6864 | Drug Info | [31], [51] | |||
| 29 | Glu-Trp-Pro-Arg-Pro-Gln-Ile-Pro-Pro | Drug Info | [52] | |||
| 30 | Iva-His-Pro-Phe-His-ACHPA-Leu-Phe-NH2 | Drug Info | [53] | |||
| 31 | Iva-His-Pro-Phe-His-AHPPA-Leu-Phe-NH2 | Drug Info | [53] | |||
| 32 | Iva-His-Pro-Phe-His-Sta-Leu-Phe-NH2 | Drug Info | [53] | |||
| 33 | N-Methyl-N-(Methylbenzyl)Formamide | Drug Info | [48] | |||
| 34 | PP1-Pro-Phe-N-MeHis-LVA-Ile-Amp-(O) | Drug Info | [55] | |||
| 35 | Pro-His-Pro-His-Leu-Phe-Val-Tyr | Drug Info | [52] | |||
| 36 | Pro-His-Pro-His-Phe-Phe-Val-Tyr | Drug Info | [52] | |||
| 37 | Pro-His-Pro-His-Phe-Phe-Val-Tyr-Lys | Drug Info | [52] | |||
| 38 | Pro-His-Pro-Phe-His-Leu(CH2NH)Val-Ile-His-Lys | Drug Info | [56] | |||
| 39 | Ro-65-7219 | Drug Info | [49] | |||
| 40 | Ro-66-1168 | Drug Info | [37] | |||
| 41 | SPP-800 | Drug Info | [57] | |||
| 42 | Sul-Pro-Phe-N-MeHis-LVA-Ile-Amp | Drug Info | [55] | |||
| 43 | Sul-Pro-Phe-N-MeHis-LVA-Ile-Amp-(O) | Drug Info | [55] | |||
| Modulator | [+] 2 Modulator drugs | + | ||||
| 1 | TAK-272 | Drug Info | [32] | |||
| 2 | JT-2724 | Drug Info | [54] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Remikiren | Ligand Info | |||||
| Structure Description | Human renin in complex with remikiren | PDB:3D91 | ||||
| Method | X-ray diffraction | Resolution | 2.20 Å | Mutation | No | [58] |
| PDB Sequence |
GNTTSSVILT
13 NYMDTQYYGE23 IGIGTPPQTF33 KVVFDTGSSN43 VWVPSSKCSR53 LYTACVYHKL 63 FDASDSSSYK73 HNGTELTLRY83 STGTVSGFLS93 QDIITVGGIT103 VTQMFGEVTE 113 MPALPFMLAE123 FDGVVGMGFI133 EQAIGRVTPI143 FDNIISQGVL153 KEDVFSFYYN 163 RDSSLGGQIV177 LGGSDPQHYE187 GNFHYINLIK197 TGVWQIQMKG207 VSVGSSTLLC 217 EDGCLALVDT227 GASYISGSTS237 SIEKLMEALG247 AKKRLFDYVV257 KCNEGPTLPD 267 ISFHLGGKEY277 TLTSADYVFQ287 ESYSSKKLCT297 LAIHAMDIPP307 PTGPTWALGA 317 TFIRKFYTEF327 DRRNNRIGFA337 LARH
|
|||||
|
|
THR18
4.055
GLN19
3.329
VAL36
4.195
ASP38
2.603
GLY40
3.322
SER41
4.520
TYR83
3.513
SER84
2.454
THR85
2.759
PRO118
3.963
PHE119
3.927
LEU121
4.424
ALA122
3.867
|
|||||
| Ligand Name: Aliskiren | Ligand Info | |||||
| Structure Description | Crystal Structure of Renin with Inhibitor 10 (Aliskiren) | PDB:2V0Z | ||||
| Method | X-ray diffraction | Resolution | 2.20 Å | Mutation | No | [59] |
| PDB Sequence |
LTLGNTTSSV
4 ILTNYMDTQY14 YGEIGIGTPP24 QTFKVVFDTG34 SSNVWVPSSK44 CSRLYTACVY 52 HKLFDASDSS62 SYKHNGTELT72 LRYSTGTVSG82 FLSQDIITVG92 GITVTQMFGE 103 VTEMPALPFM113 LAEFDGVVGM123 GFIEQAIGRV133 TPIFDNIISQ143 GVLKEDVFSF 153 YYNRDSESQS160D LGGQIVLGGS170 DPQHYEGNFH180 YINLIKTGVW190 QIQMKGVSVG 200 SSTLLCEDGC210 LALVDTGASY220 ISGSTSSIEK230 LMEALGAKKR240 LFDYVVKCNE 251 GPTLPDISFH261 LGGKEYTLTS271 ADYVFQESYS281 SKKLCTLAIH287 AMDIPPPTGP 297 TWALGATFIR307 KFYTEFDRRN317 NRIGFALAR
|
|||||
|
|
THR12
3.251
GLN13
3.340
TYR14
3.013
VAL30
3.493
ASP32
2.710
GLY34
2.984
SER35
3.636
ARG74
2.985
TYR75
3.142
SER76
2.896
THR77
3.862
PRO111
3.893
PHE112
4.479
LEU114
3.738
ALA115
3.811
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Renin-angiotensin system | hsa04614 | Affiliated Target |
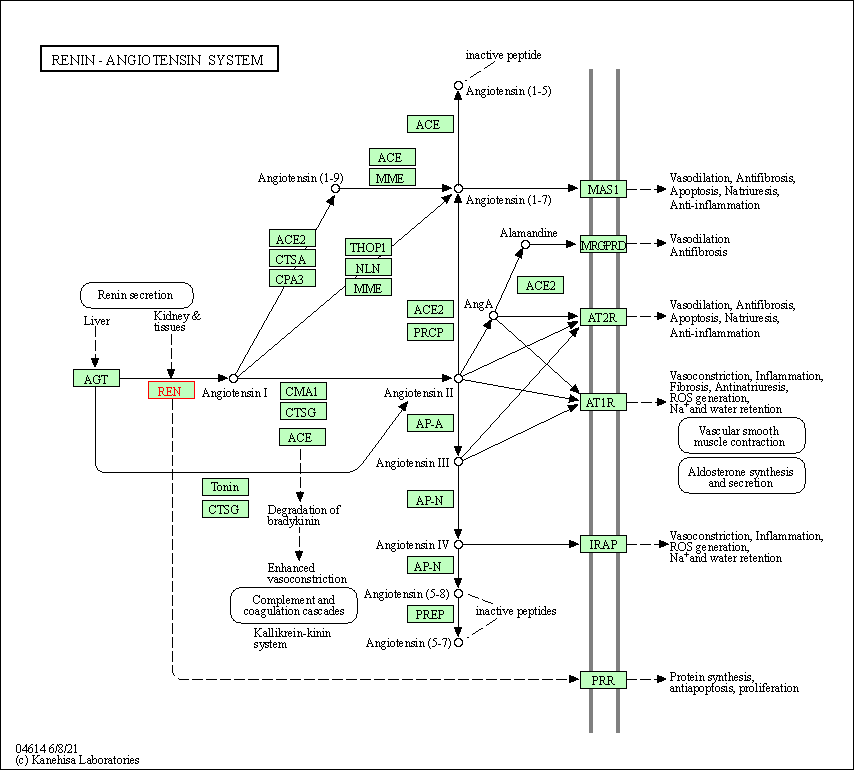
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Renin secretion | hsa04924 | Affiliated Target |
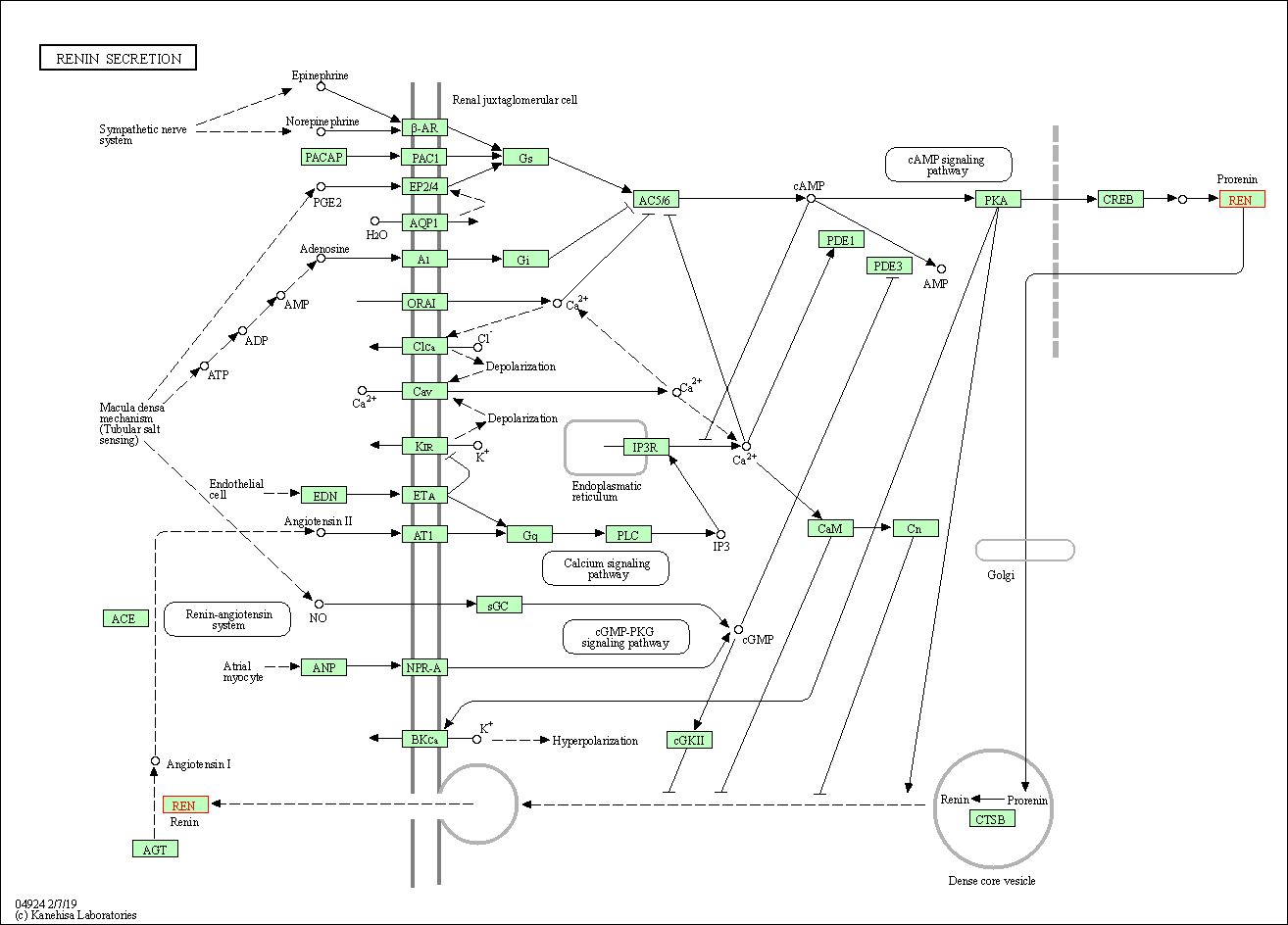
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 6 | Degree centrality | 6.45E-04 | Betweenness centrality | 1.56E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.90E-01 | Radiality | 1.32E+01 | Clustering coefficient | 2.00E-01 |
| Neighborhood connectivity | 8.50E+00 | Topological coefficient | 1.99E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 1 KEGG Pathways | + | ||||
| 1 | Renin-angiotensin system | |||||
| Pathwhiz Pathway | [+] 1 Pathwhiz Pathways | + | ||||
| 1 | Angiotensin Metabolism | |||||
| Reactome | [+] 1 Reactome Pathways | + | ||||
| 1 | Metabolism of Angiotensinogen to Angiotensins | |||||
| WikiPathways | [+] 2 WikiPathways | + | ||||
| 1 | ACE Inhibitor Pathway | |||||
| 2 | Metabolism of Angiotensinogen to Angiotensins | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Comparative effects of aliskiren-based and ramipril-based therapy on the renin system during long-term (6 months) treatment and withdrawal in patie... J Renin Angiotensin Aldosterone Syst. 2009 Sep;10(3):157-67. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4812). | |||||
| REF 3 | 2007 FDA drug approvals: a year of flux. Nat Rev Drug Discov. 2008 Feb;7(2):107-9. | |||||
| REF 4 | Agents in development for the treatment of diabetic nephropathy. Expert Opin Emerg Drugs. 2008 Sep;13(3):447-63. | |||||
| REF 5 | Functional expression of the renin-angiotensin system in human podocytes. Am J Physiol Renal Physiol. 2006 Mar;290(3):F710-9. | |||||
| REF 6 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001570) | |||||
| REF 7 | ClinicalTrials.gov (NCT05770609) A Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel, Dose-finding Phase II Clinical Study to Evaluate the Efficacy and Safety of SPH3127 Tablets in the Treatment of Mild to Moderate Ulcerative Colitis. U.S.National Institutes of Health. | |||||
| REF 8 | ClinicalTrials.gov (NCT00376636) Phase IIa Safety and Efficacy Study of SPP635 in Mild to Moderate Hypertension. U.S. National Institutes of Health. | |||||
| REF 9 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001290) | |||||
| REF 10 | ClinicalTrials.gov (NCT02332824) A Phase 2 Dose-finding Study of TAK-272 in Patients With Type 2 Diabetes Mellitus and Microalbuminuria. U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT01757860) Safety and Pharmacokinetics Study of CARD-024 in Healthy Subjects. U.S. National Institutes of Health. | |||||
| REF 12 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800029409) | |||||
| REF 13 | ClinicalTrials.gov (NCT01217736) Direct Renin Inhibition and the Kidney. U.S. National Institutes of Health. | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000369) | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001939) | |||||
| REF 16 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002065) | |||||
| REF 17 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000425) | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025565) | |||||
| REF 19 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800027192) | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002396) | |||||
| REF 21 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001791) | |||||
| REF 22 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006753) | |||||
| REF 23 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005529) | |||||
| REF 24 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002295) | |||||
| REF 25 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010164) | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005981) | |||||
| REF 27 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004067) | |||||
| REF 28 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002406) | |||||
| REF 29 | Effect of SPP 635, a renin inhibitor, on intraocular pressure in glaucomatous monkey eyes. Exp Eye Res. 2012 Jan;94(1):146-9. | |||||
| REF 30 | Effects of a renin inhibitor, SR 43845, and of captopril on blood pressure and plasma active renin in conscious sodium-replete macaca. J Hypertens Suppl. 1989 Apr;7(2):S33-5. | |||||
| REF 31 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 32 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800033655) | |||||
| REF 33 | Drug-drug interaction study of ACT-178882, a new renin inhibitor, and diltiazem in healthy subjects. Clin Drug Investig. 2013 Mar;33(3):207-13. | |||||
| REF 34 | New Developments in the Pharmacological Treatment of Hypertension: Dead-End or a Glimmer at the Horizon . Curr Hypertens Rep. 2015; 17(6): 42. | |||||
| REF 35 | Comparative effects of three different potent renin inhibitors in primates. Hypertension. 1993 Jul;22(1):9-17. | |||||
| REF 36 | Antihypertensive efficacy of FK906, a novel human renin inhibitor. Clin Ther. 1993 May-Jun;15(3):539-48. | |||||
| REF 37 | Design and preparation of potent, nonpeptidic, bioavailable renin inhibitors. J Med Chem. 2009 Jun 25;52(12):3689-702. | |||||
| REF 38 | The orally active renin inhibitor A-74273. In vivo and in vitro morpholine ring metabolism in rats, dogs, and humans. Drug Metab Dispos. 1994 Nov-Dec;22(6):880-8. | |||||
| REF 39 | Comparative studies on differential inhibition of the renin - angiotensin system in the anesthetized guinea pig. Can J Physiol Pharmacol. 1995 Oct;73(10):1512-8. | |||||
| REF 40 | Ciprokiren (Ro 44-9375). A renin inhibitor with increasing effects on chronic treatment. Hypertension. 1994 Aug;24(2):163-9. | |||||
| REF 41 | The effect of the renin inhibitor ES-1005 on the expression of the kidney renin gene in sodium-depleted marmosets. J Hypertens. 1990 Dec;8(12):1143-6. | |||||
| REF 42 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010026) | |||||
| REF 43 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005981) | |||||
| REF 44 | Effects of SC-56525, a potent, orally active renin inhibitor, in salt-depleted and renal hypertensive dogs. Hypertension. 1995 Jul;26(1):95-100. | |||||
| REF 45 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002406) | |||||
| REF 46 | Renin inhibitors. Dipeptide analogues of angiotensinogen incorporating transition-state, nonpeptidic replacements at the scissile bond. J Med Chem. 1987 Oct;30(10):1729-37. | |||||
| REF 47 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 48 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 49 | Direct renin inhibitors as a new therapy for hypertension. J Med Chem. 2010 Nov 11;53(21):7490-520. | |||||
| REF 50 | Liver disease associated with occupational exposure to the solvent dimethylformamide. Ann Intern Med. 1988 May;108(5):680-6. | |||||
| REF 51 | A highly potent and long-acting oral inhibitor of human renin. Hypertension. 1988 Jun;11(6 Pt 2):708-12. | |||||
| REF 52 | Inhibition of the renin-angiotensin system. A new approach to the therapy of hypertension. J Med Chem. 1981 Apr;24(4):355-61. | |||||
| REF 53 | Renin inhibitors. Syntheses of subnanomolar, competitive, transition-state analogue inhibitors containing a novel analogue of statine. J Med Chem. 1985 Dec;28(12):1779-90. | |||||
| REF 54 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | |||||
| REF 55 | Renin inhibitory peptides. Incorporation of polar, hydrophilic end groups into an active renin inhibitory peptide template and their evaluation in ... J Med Chem. 1991 Feb;34(2):633-42. | |||||
| REF 56 | Synthesis and biological activity of some transition-state inhibitors of human renin. J Med Chem. 1988 Sep;31(9):1839-46. | |||||
| REF 57 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2413). | |||||
| REF 58 | Human renin in complex with remikiren | |||||
| REF 59 | Structure-based drug design: the discovery of novel nonpeptide orally active inhibitors of human renin. Chem Biol. 2000 Jul;7(7):493-504. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

