Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T68782
(Former ID: TTDR00802)
|
|||||
| Target Name |
Prostaglandin D2 receptor (PTGDR)
|
|||||
| Synonyms |
Prostanoid DP receptor; PGD2 receptor; PGD receptor
Click to Show/Hide
|
|||||
| Gene Name |
PTGDR
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Coronary atherosclerosis [ICD-11: BA80] | |||||
| Function |
The activity of this receptor is mainly mediated by G(s) proteins that stimulate adenylate cyclase, resulting in an elevation of intracellular cAMP. A mobilization of calcium is also observed, but without formation of inositol 1,4,5-trisphosphate. Receptor for prostaglandin D2 (PGD2).
Click to Show/Hide
|
|||||
| BioChemical Class |
GPCR rhodopsin
|
|||||
| UniProt ID | ||||||
| Sequence |
MKSPFYRCQNTTSVEKGNSAVMGGVLFSTGLLGNLLALGLLARSGLGWCSRRPLRPLPSV
FYMLVCGLTVTDLLGKCLLSPVVLAAYAQNRSLRVLAPALDNSLCQAFAFFMSFFGLSST LQLLAMALECWLSLGHPFFYRRHITLRLGALVAPVVSAFSLAFCALPFMGFGKFVQYCPG TWCFIQMVHEEGSLSVLGYSVLYSSLMALLVLATVLCNLGAMRNLYAMHRRLQRHPRSCT RDCAEPRADGREASPQPLEELDHLLLLALMTVLFTMCSLPVIYRAYYGAFKDVKEKNRTS EEAEDLRALRFLSVISIVDPWIFIIFRSPVFRIFFHKIFIRPLRYRSRCSNSTNMESSL Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 9 Clinical Trial Drugs | + | ||||
| 1 | QAW-039 | Drug Info | Phase 3 | Asthma | [3] | |
| 2 | Setipiprant | Drug Info | Phase 3 | Asthma | [4] | |
| 3 | MK-1029 | Drug Info | Phase 2 | Asthma | [6] | |
| 4 | ONO-4053 | Drug Info | Phase 2 | Allergic rhinitis | [7] | |
| 5 | S-555739 | Drug Info | Phase 2 | Allergy | [8] | |
| 6 | TS-022 | Drug Info | Phase 2 | Atopic dermatitis | [9] | |
| 7 | 192C86 | Drug Info | Phase 1 | Thrombosis | [11] | |
| 8 | SAR-389644 | Drug Info | Phase 1 | Allergic rhinitis | [12] | |
| 9 | PGF2alpha | Drug Info | Clinical trial | Solid tumour/cancer | [13] | |
| Discontinued Drug(s) | [+] 4 Discontinued Drugs | + | ||||
| 1 | Laropiprant+niacin | Drug Info | Discontinued in Phase 3 | Dyslipidemia | [14] | |
| 2 | S-5751 | Drug Info | Discontinued in Phase 2 | Rhinitis | [15], [16] | |
| 3 | ONO-4127 | Drug Info | Discontinued in Phase 1 | Allergic rhinitis | [17] | |
| 4 | ZK-118182 | Drug Info | Terminated | Thrombosis | [18], [19] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Antagonist | [+] 11 Antagonist drugs | + | ||||
| 1 | QAW-039 | Drug Info | [20] | |||
| 2 | Setipiprant | Drug Info | [21] | |||
| 3 | ONO-4053 | Drug Info | [23] | |||
| 4 | S-555739 | Drug Info | [8] | |||
| 5 | SAR-389644 | Drug Info | [26] | |||
| 6 | Laropiprant+niacin | Drug Info | [28] | |||
| 7 | S-5751 | Drug Info | [29] | |||
| 8 | ONO-4127 | Drug Info | [30] | |||
| 9 | AH6809 | Drug Info | [27] | |||
| 10 | BWA868C | Drug Info | [32], [34] | |||
| 11 | ONO-AE3-237 | Drug Info | [36] | |||
| Inhibitor | [+] 2 Inhibitor drugs | + | ||||
| 1 | MK-1029 | Drug Info | [22] | |||
| 2 | DIASTEREOMER 2 | Drug Info | [1] | |||
| Agonist | [+] 13 Agonist drugs | + | ||||
| 1 | TS-022 | Drug Info | [24] | |||
| 2 | PGF2alpha | Drug Info | [27] | |||
| 3 | 15-deoxy-Delta12,14-PGJ2 | Drug Info | [32] | |||
| 4 | butaprost (free acid form) | Drug Info | [27] | |||
| 5 | BW 245C | Drug Info | [33] | |||
| 6 | cloprostenol | Drug Info | [27] | |||
| 7 | Delta12-PGJ2 | Drug Info | [32] | |||
| 8 | L-644,698 | Drug Info | [35] | |||
| 9 | PGD2 | Drug Info | [35], [37] | |||
| 10 | RS 93520 | Drug Info | [38] | |||
| 11 | SQ-27986 | Drug Info | [38] | |||
| 12 | U46619 | Drug Info | [27] | |||
| 13 | ZK110841 | Drug Info | [32] | |||
| Modulator | [+] 3 Modulator drugs | + | ||||
| 1 | 192C86 | Drug Info | [25] | |||
| 2 | ZK-118182 | Drug Info | [31] | |||
| 3 | TTH 03-001 | Drug Info | [39] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Human Similarity Proteins
Human Pathway Affiliation
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| Olfactory receptor 10H3 (OR10H3) | 25.455 (28/110) | 2.00E-03 | |
| Olfactory receptor 10H5 (OR10H5) | 27.211 (40/147) | 3.00E-03 |
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Neuroactive ligand-receptor interaction | hsa04080 | Affiliated Target |
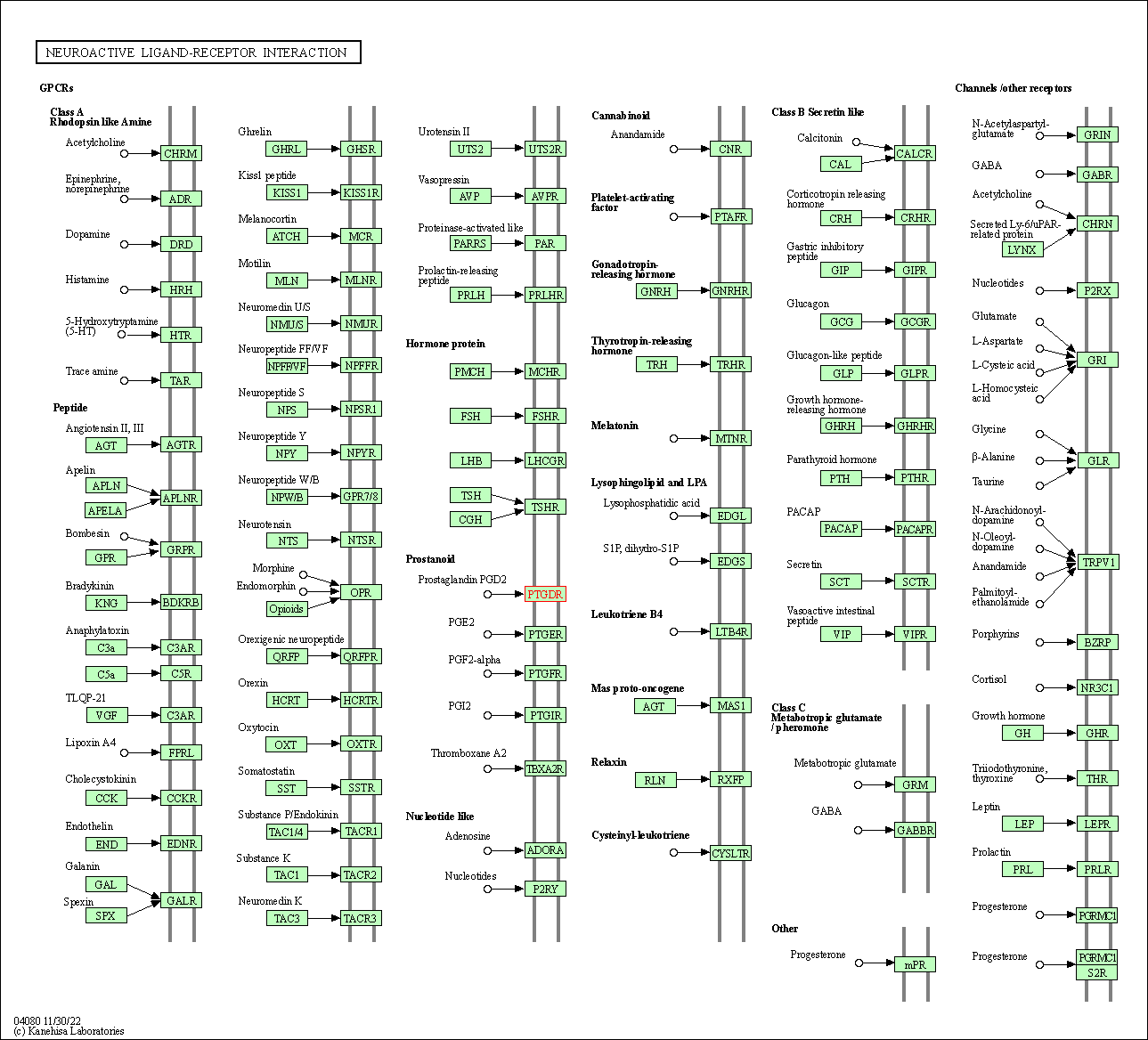
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 1 KEGG Pathways | + | ||||
| 1 | Neuroactive ligand-receptor interaction | |||||
| Pathwhiz Pathway | [+] 1 Pathwhiz Pathways | + | ||||
| 1 | Intracellular Signalling Through PGD2 receptor and Prostaglandin D2 | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | Thromboxane A2 receptor signaling | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Prostanoid ligand receptors | |||||
| 2 | G alpha (s) signalling events | |||||
| WikiPathways | [+] 5 WikiPathways | + | ||||
| 1 | Prostaglandin Synthesis and Regulation | |||||
| 2 | GPCRs, Class A Rhodopsin-like | |||||
| 3 | Small Ligand GPCRs | |||||
| 4 | GPCR ligand binding | |||||
| 5 | GPCR downstream signaling | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydroc... J Med Chem. 2007 Feb 22;50(4):794-806. | |||||
| REF 2 | ClinicalTrials.gov (NCT01126073) A Double Blind, Randomized Study to Compare Influence of Niacin/Laropiprant on Functional and Morphological Characteristics of Arterial Wall and Parameters of Inflammation in Subjects With Coronary Heart Disease Already Treated With a Statin in Miran Sebestjen, University Medical Centre Ljubljana. | |||||
| REF 3 | ClinicalTrials.gov (NCT02563067) Study of Efficacy and Safety of QAW039 in Patients With Severe Asthma Inadequately Controlled With Standard of Care Asthma Treatment. | |||||
| REF 4 | ClinicalTrials.gov (NCT01484119) Efficacy, Safety, and Tolerability Study of ACT-129968 in Patients With Seasonal Allergic Rhinitis. U.S. National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT01018550) AMG 853 Phase 2 Study in Subjects With Inadequately Controlled Asthma. U.S. National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT01624974) Study to Evaluate the Effectiveness and Safety of MK-1029 in the Treatment of Persistent Asthma That is Not Controlled With Montelukast (ML) in Adults (MK-1029-011 AM2). U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT01748344) Phase II Study Evaluating ONO-4053 and Cetirizine in Subjects With Seasonal Allergic Rhinitis. U.S. National Institutes of Health. | |||||
| REF 8 | Clinical pipeline report, company report or official report of Shionogi (2011). | |||||
| REF 9 | ClinicalTrials.gov (NCT00914186) Study of TS-022 in Adult Patients With Atopic Dermatitis With Pruritus (POC). U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT03170544) Single Ascending Dose Study of MK-1092 in Healthy Participants and in Participants With Type 1 and Type 2 Diabetes Mellitus (MK-1092-001). U.S. National Institutes of Health. | |||||
| REF 11 | The effect of a prostaglandin DP-receptor partial agonist (192C86) on platelet aggregation and the cardiovascular system in healthy volunteers. Br J Clin Pharmacol. 1992 Oct;34(4):344-51. | |||||
| REF 12 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025865) | |||||
| REF 13 | Stereocontrolled organocatalytic synthesis of prostaglandin PGF2alpha in seven steps. Nature. 2012 Sep 13;489(7415):278-81. | |||||
| REF 14 | ClinicalTrials.gov (NCT01054508) Effect of Tredaptive on Serum Lipoproteins and Inflammatory Markers. U.S. National Institutes of Health. | |||||
| REF 15 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1898). | |||||
| REF 16 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800012090) | |||||
| REF 17 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018932) | |||||
| REF 18 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1890). | |||||
| REF 19 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003331) | |||||
| REF 20 | Effect Of QAW039, An Oral Prostaglandin D2 Receptor (DP2/CRTh2) Antagonist, Upon Sputum And Bronchial Eosinophilic Inflammation And Clinical Outcomes In Treatment-Resistant Asthma: A Phase 2a Randomized Placebo-Controlled Trial. American Thoracic Society Journals. 2015. | |||||
| REF 21 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 22 | Update on the status of DP2 receptor antagonists; from proof of concept through clinical failures to promising new drugs. Expert Opin Investig Drugs. 2014 Jan;23(1):55-66. | |||||
| REF 23 | Effect of ONO-4053, a DP1 (prostaglandin D2 receptor) Antagonist, on Prostaglandin D2-Induced Nasal Congestion. Journal of Allergy and Clinical Immunology Volume 135, Issue 2, Supplement, February 2015, Pages AB219. | |||||
| REF 24 | Effects of TS-022, a newly developed prostanoid DP1 receptor agonist, on experimental pruritus, cutaneous barrier disruptions and atopic dermatitis... Eur J Pharmacol. 2007 Feb 5;556(1-3):207-14. | |||||
| REF 25 | Prostanoid receptor antagonists: development strategies and therapeutic applications. Br J Pharmacol. 2009 September; 158(1): 104-145. | |||||
| REF 26 | US patent application no. 2008,0300,217, Combination therapy with fumaric acid esters for the treatment of autoimmune and,or inflammatory disorders. | |||||
| REF 27 | The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000 Jan 17;1483(2):285-93. | |||||
| REF 28 | Emerging antidyslipidemic drugs. Expert Opin Emerg Drugs. 2008 Jun;13(2):363-81. | |||||
| REF 29 | Therapeutic target database update 2012: a resource for facilitating target-oriented drug discovery. Nucleic Acids Res. 2012 Jan;40(Database issue):D1128-36. | |||||
| REF 30 | Sleep-wake regulation by prostaglandin D2 and adenosine. Brain Nerve. 2012 Jun;64(6):621-8. | |||||
| REF 31 | Characterization of prostanoid receptors mediating inhibition of histamine release from anti-IgE-activated rat peritoneal mast cells. Br J Pharmacol. 2000 Feb;129(3):589-97. | |||||
| REF 32 | Characterization of the recombinant human prostanoid DP receptor and identification of L-644,698, a novel selective DP agonist. Br J Pharmacol. 1998 Apr;123(7):1317-24. | |||||
| REF 33 | Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997 Sep;122(2):217-24. | |||||
| REF 34 | Pharmacology and autoradiography of human DP prostanoid receptors using [(3)H]-BWA868C, a DP receptor-selective antagonist radioligand. Br J Pharmacol. 2000 Nov;131(6):1025-38. | |||||
| REF 35 | A novel biological role for prostaglandin D2 is suggested by distribution studies of the rat DP prostanoid receptor. Eur J Pharmacol. 1999 Jul 14;377(1):101-15. | |||||
| REF 36 | Prostaglandin D2 inhibits the production of IFN-gamma by invariant NK T cells: consequences in the control of B16 melanoma. J Immunol. 2008 Jan 15;180(2):783-92. | |||||
| REF 37 | GW627368X ((N-{2-[4-(4,9-diethoxy-1-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl]acetyl} benzene sulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. Br J Pharmacol. 2006 Jun;148(3):326-39. | |||||
| REF 38 | Affinities, selectivities, potencies, and intrinsic activities of natural and synthetic prostanoids using endogenous receptors: focus on DP class prostanoids. J Pharmacol Exp Ther. 2000 May;293(2):321-8. | |||||
| REF 39 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 338). | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

