Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T73215
(Former ID: TTDS00268)
|
|||||
| Target Name |
Leukocyte surface antigen Leu-16 (CD20)
|
|||||
| Synonyms |
Membrane-spanning 4-domains subfamily A member 1; Leu-16; Bp35; B-lymphocyte surface antigen B1; B-lymphocyte antigen CD20
Click to Show/Hide
|
|||||
| Gene Name |
MS4A1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 6 Target-related Diseases | + | ||||
| 1 | Diffuse large B-cell lymphoma [ICD-11: 2A81] | |||||
| 2 | Follicular lymphoma [ICD-11: 2A80] | |||||
| 3 | Malignant haematopoietic neoplasm [ICD-11: 2B33] | |||||
| 4 | Mature B-cell leukaemia [ICD-11: 2A82] | |||||
| 5 | Mature B-cell lymphoma [ICD-11: 2A85] | |||||
| 6 | Multiple sclerosis [ICD-11: 8A40] | |||||
| Function |
Functions as a store-operated calcium (SOC) channel component promoting calcium influx after activation by the B-cell receptor/BCR. B-lymphocyte-specific membrane protein that plays a role in the regulation of cellular calcium influx necessary for the development, differentiation, and activation of B-lymphocytes.
Click to Show/Hide
|
|||||
| BioChemical Class |
CD20 calcium channel
|
|||||
| UniProt ID | ||||||
| Sequence |
MTTPRNSVNGTFPAEPMKGPIAMQSGPKPLFRRMSSLVGPTQSFFMRESKTLGAVQIMNG
LFHIALGGLLMIPAGIYAPICVTVWYPLWGGIMYIISGSLLAATEKNSRKCLVKGKMIMN SLSLFAAISGMILSIMDILNIKISHFLKMESLNFIRAHTPYINIYNCEPANPSEKNSPST QYCYSIQSLFLGILSVMLIFAFFQELVIAGIVENEWKRTCSRPKSNIVLLSAEEKKEQTI EIKEEVVGLTETSSQPKNEEDIEIIPIQEEEEEETETNFPEPPQDQESSPIENDSSP Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T11Z2G | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 6 Approved Drugs | + | ||||
| 1 | Ibritumomab | Drug Info | Approved | Non-hodgkin lymphoma | [9] | |
| 2 | Obinutuzumab | Drug Info | Approved | Chronic lymphocytic leukaemia | [10], [11] | |
| 3 | Rituximab | Drug Info | Approved | Non-hodgkin lymphoma | [12], [13] | |
| 4 | Tisagenlecleucel | Drug Info | Approved | Acute lymphoblastic leukaemia | [14] | |
| 5 | Tositumomab | Drug Info | Approved | Non-hodgkin lymphoma | [9], [15] | |
| 6 | Ublituximab | Drug Info | Approved | Multiple sclerosis | [16] | |
| Clinical Trial Drug(s) | [+] 31 Clinical Trial Drugs | + | ||||
| 1 | Bevacizumab + Rituximab | Drug Info | Phase 3 | Non-hodgkin lymphoma | [18] | |
| 2 | MK-8808 | Drug Info | Phase 3 | Follicular lymphoma | [19] | |
| 3 | PF-05280586 | Drug Info | Phase 3 | Cluster headache | [20] | |
| 4 | Ublituximab + umbralisib | Drug Info | Phase 3 | Chronic lymphocytic leukaemia | [21] | |
| 5 | AME-133v | Drug Info | Phase 2 | Non-hodgkin lymphoma | [27], [28], [29] | |
| 6 | ATM AVI | Drug Info | Phase 2 | Bacterial infection | [30] | |
| 7 | CAR-T cells targeting CD20 | Drug Info | Phase 2 | Non-hodgkin lymphoma | [31] | |
| 8 | DI-Leu16-IL2 | Drug Info | Phase 2 | Non-hodgkin lymphoma | [21] | |
| 9 | REGN1979 | Drug Info | Phase 2 | B-cell non-hodgkin lymphoma | [32] | |
| 10 | TRU-015 | Drug Info | Phase 2 | Lymphoma | [33] | |
| 11 | Veltuzumab | Drug Info | Phase 2 | Systemic lupus erythematosus | [34], [35] | |
| 12 | 4SCAR19 and 4SCAR20 | Drug Info | Phase 1/2 | B-cell lymphoma | [39] | |
| 13 | Anti-CD19 and Anti-CD20 CAR-T Cells | Drug Info | Phase 1/2 | B-cell lymphoma | [40] | |
| 14 | Anti-CD20 CAR-T cells | Drug Info | Phase 1/2 | Diffuse large B-cell lymphoma | [41] | |
| 15 | Anti-CD20-CAR-transduced T cells | Drug Info | Phase 1/2 | leukaemia | [42] | |
| 16 | CART20 | Drug Info | Phase 1/2 | B-cell lymphoma | [43] | |
| 17 | CD19 and CD20 CAR-T Cells | Drug Info | Phase 1/2 | B-cell lymphoma | [44] | |
| 18 | CD20-Specific CAR T Cells | Drug Info | Phase 1/2 | Chronic lymphocytic leukaemia | [45] | |
| 19 | FBT-A05 | Drug Info | Phase 1/2 | Chronic lymphocytic leukaemia | [46] | |
| 20 | MB-CART20.1 | Drug Info | Phase 1/2 | Non-hodgkin lymphoma | [47] | |
| 21 | Anti-CD19 anti-CD20 Bispecific CAR-T | Drug Info | Phase 1 | leukaemia | [52] | |
| 22 | BM-ca | Drug Info | Phase 1 | Non-hodgkin lymphoma | [53] | |
| 23 | CAR-20/19-T Cells | Drug Info | Phase 1 | B-cell non-hodgkin lymphoma | [54] | |
| 24 | CART-20 cells | Drug Info | Phase 1 | Acute lymphoblastic leukaemia | [55] | |
| 25 | CD20-CAR-T Cells | Drug Info | Phase 1 | B-cell lymphoma | [56] | |
| 26 | CD20-CART | Drug Info | Phase 1 | leukaemia | [57] | |
| 27 | CD20Bi aATC | Drug Info | Phase 1 | Non-hodgkin lymphoma | [58] | |
| 28 | Iboctadekin + rituximab | Drug Info | Phase 1 | Follicular lymphoma | [59] | |
| 29 | RG7828 | Drug Info | Phase 1 | Haematological malignancy | [60] | |
| 30 | XmAb13676 | Drug Info | Phase 1 | B-cell lymphoma | [21] | |
| 31 | Anti-CD19/20-CAR vector-transduced T cells | Drug Info | Clinical trial | Acute lymphoblastic leukaemia | [61] | |
| Mode of Action | [+] 8 Modes of Action | + | ||||
| Immunostimulant | [+] 1 Immunostimulant drugs | + | ||||
| 1 | Tisagenlecleucel | Drug Info | [60] | |||
| Agonist | [+] 1 Agonist drugs | + | ||||
| 1 | MK-8808 | Drug Info | [68] | |||
| Antagonist | [+] 2 Antagonist drugs | + | ||||
| 1 | PF-05280586 | Drug Info | [69] | |||
| 2 | TRU-015 | Drug Info | [74] | |||
| Modulator | [+] 8 Modulator drugs | + | ||||
| 1 | ATM AVI | Drug Info | [64] | |||
| 2 | DI-Leu16-IL2 | Drug Info | [71] | |||
| 3 | Veltuzumab | Drug Info | [75] | |||
| 4 | CD20Bi aATC | Drug Info | [58] | |||
| 5 | 2LM20-4 | Drug Info | [64] | |||
| 6 | Anti-CD20 engineered toxin bodies | Drug Info | [64] | |||
| 7 | DXL-625 | Drug Info | [64] | |||
| 8 | MEDI-552 | Drug Info | [79] | |||
| CAR-T-Cell-Therapy | [+] 7 CAR-T-Cell-Therapy drugs | + | ||||
| 1 | CAR-T cells targeting CD20 | Drug Info | [31] | |||
| 2 | Anti-CD20-CAR-transduced T cells | Drug Info | [42] | |||
| 3 | CART20 | Drug Info | [43] | |||
| 4 | CD20-Specific CAR T Cells | Drug Info | [45] | |||
| 5 | MB-CART20.1 | Drug Info | [47] | |||
| 6 | CART-20 cells | Drug Info | [55] | |||
| 7 | CD20-CAR-T Cells | Drug Info | [56] | |||
| Inhibitor | [+] 2 Inhibitor drugs | + | ||||
| 1 | REGN1979 | Drug Info | [60] | |||
| 2 | Anti-CD-20 mab | Drug Info | [64] | |||
| CAR-T-Cell-Therapy(Dual specific) | [+] 8 CAR-T-Cell-Therapy(Dual specific) drugs | + | ||||
| 1 | 4SCAR19 and 4SCAR20 | Drug Info | [39] | |||
| 2 | Anti-CD19 and Anti-CD20 CAR-T Cells | Drug Info | [40] | |||
| 3 | Anti-CD20 CAR-T cells | Drug Info | [41] | |||
| 4 | CD19 and CD20 CAR-T Cells | Drug Info | [44] | |||
| 5 | Anti-CD19 anti-CD20 Bispecific CAR-T | Drug Info | [52] | |||
| 6 | CAR-20/19-T Cells | Drug Info | [54] | |||
| 7 | CD20-CART | Drug Info | [57] | |||
| 8 | Anti-CD19/20-CAR vector-transduced T cells | Drug Info | [61] | |||
| Stimulator | [+] 1 Stimulator drugs | + | ||||
| 1 | RG7828 | Drug Info | [60] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Cholesterol hemisuccinate | Ligand Info | |||||
| Structure Description | Structure of CD20 in complex with rituximab Fab | PDB:6VJA | ||||
| Method | Electron microscopy | Resolution | 3.30 Å | Mutation | No | [80] |
| PDB Sequence |
MRESKTLGAV
55 QIMNGLFHIA65 LGGLLMIPAG75 IYAPICVTVW85 YPLWGGIMYI95 ISGSLLAATE 105 KNSRKCLVKG115 KMIMNSLSLF125 AAISGMILSI135 MDILNIKISH145 FLKMESLNFI 155 RAHTPYINIY165 NCEPANPSEK175 NSPSTQYCYS185 IQSLFLGILS195 VMLIFAFFQE 205 LVIAG
|
|||||
|
|
ILE64
4.284
GLY67
4.497
GLY68
3.765
MET71
3.475
ILE72
4.292
PRO73
3.804
TRP85
4.277
PRO87
4.918
LEU88
4.046
MET93
3.858
ILE96
3.651
GLY115
4.040
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: Pentadecane | Ligand Info | |||||
| Structure Description | Structure of full-length CD20 in complex with Rituximab Fab | PDB:6Y90 | ||||
| Method | Electron microscopy | Resolution | 3.69 Å | Mutation | No | [81] |
| PDB Sequence |
FMRESKTLGA
54 VQIMNGLFHI64 ALGGLLMIPA74 GIYAPICVTV84 WYPLWGGIMY94 IISGSLLAAT 104 EKNSRKCLVK114 GKMIMNSLSL124 FAAISGMILS134 IMDILNIKIS144 HFLKMESLNF 154 IRAHTPYINI164 YNCEPANPSE174 KNSPSTQYCY184 SIQSLFLGIL194 SVMLIFAFFQ 204 ELVIAGIVEN214 EW
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Hematopoietic cell lineage | hsa04640 | Affiliated Target |
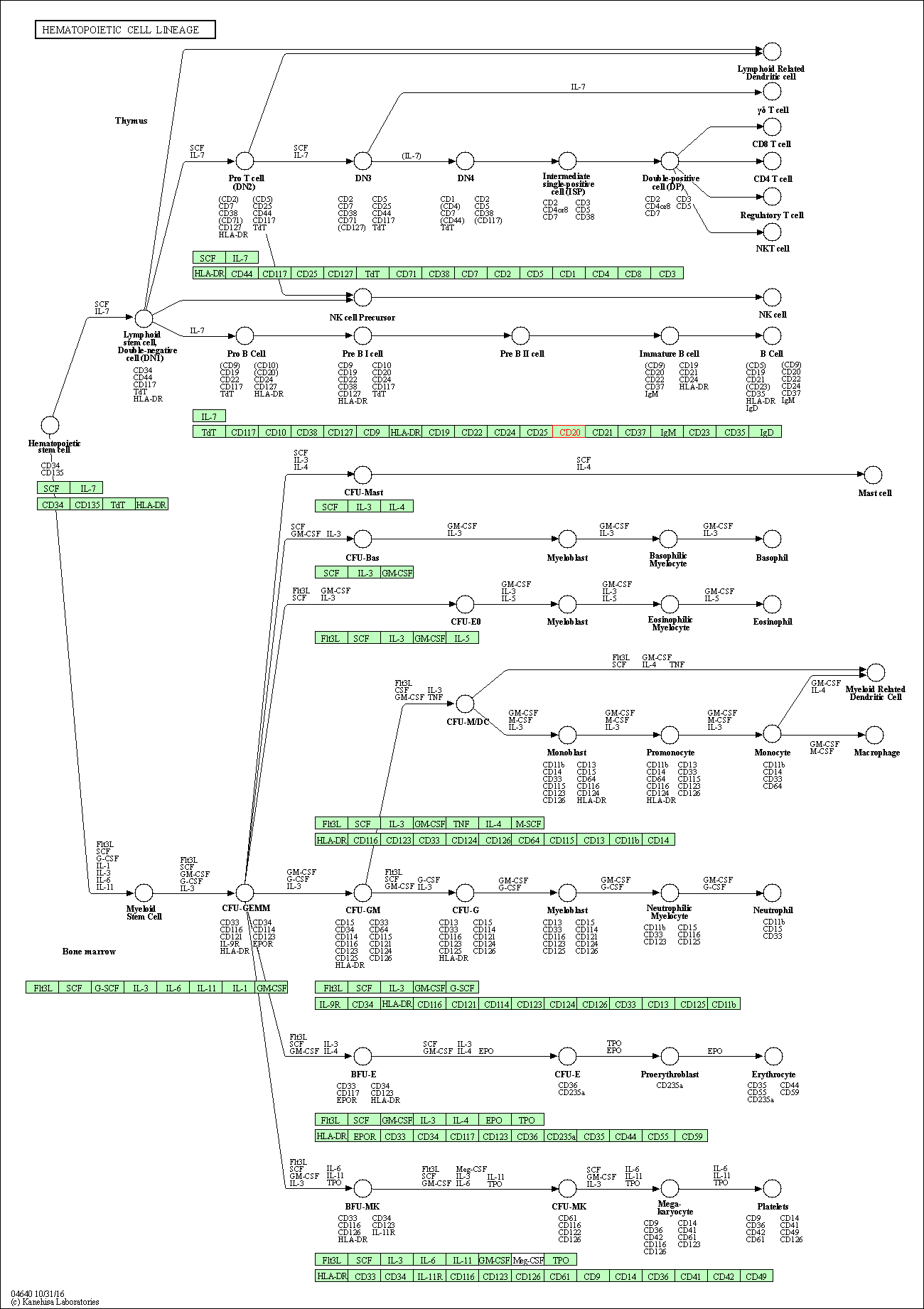
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 1 KEGG Pathways | + | ||||
| 1 | Hematopoietic cell lineage | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | [Contribution of radioimmunotherapy to the treatment of lymphoma]. Ann Pharm Fr. 2008 Nov-Dec;66(5-6):300-8. | |||||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 761324. | |||||
| REF 3 | FDA Approved Drug Products from FDA Official Website. 2022. Application Number: 761263. | |||||
| REF 4 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 761309 | |||||
| REF 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2018 | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6778). | |||||
| REF 7 | Hughes B: 2009 FDA drug approvals. Nat Rev Drug Discov. 2010 Feb;9(2):89-92. | |||||
| REF 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 9 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 10 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6941). | |||||
| REF 11 | Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. 2017 Sep 6;11:2643-2651. | |||||
| REF 12 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6780). | |||||
| REF 13 | Disease-modifying agents for multiple sclerosis: recent advances and future prospects. Drugs. 2008;68(17):2445-68. | |||||
| REF 14 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||||
| REF 15 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6781). | |||||
| REF 16 | FDA Approved Drug Products from FDA Official Website. 2022. Application Number: 761238. | |||||
| REF 17 | ClinicalTrials.gov (NCT02809053) A Randomized, Double-blind, Multi-center, Multi-national Trial to Evaluate the Efficacy, Safety, and Immunogenicity of SAIT101 Versus Rituximab as a First-line Immunotherapy Treatment in Patients With Low Tumor Burden Follicular Lymphoma (RAMO-2). U.S. National Institutes of Health. | |||||
| REF 18 | ClinicalTrials.gov (NCT00486759) A Study of Bevacizumab (Avastin) in Combination With Rituximab (MabThera) and CHOP (Cyclophosphamide, Hydroxydaunorubicin [Doxorubicin], Oncovin [Vincristine], Prednisone) Chemotherapy in Patients With Diffuse Large B-cell Lymphoma. U.S. National Institutes of Health. | |||||
| REF 19 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800034367) | |||||
| REF 20 | ClinicalTrials.gov (NCT02213263) A Study Of PF-05280586 (Rituximab-Pfizer) Or MabThera (Rituximab-EU) For The First-Line Treatment Of Patients With CD20-Positive, Low Tumor Burden, Follicular Lymphoma (REFLECTIONS B328-06). U.S. National Institutes of Health. | |||||
| REF 21 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 22 | ClinicalTrials.gov (NCT01008852) Study Evaluating The Efficacy And Safety Of SBI-087 In Seropositive Subjects With Active Rheumatoid Arthritis. U.S. National Institutes of Health. | |||||
| REF 23 | ClinicalTrials.gov (NCT02846584) a Clinical Research of Sequential CAR-T Bridging HSCT in the Treatment of Relapse/Refractory B-cell Malignancies | |||||
| REF 24 | ClinicalTrials.gov (NCT03488251) PK,PD,Safety and Tolerability of Multiple Dose Regimens of MT-3724 With Gemcitabine and Oxaliplatin for the Treatment of Patients With Relapsed/Refractory Diffuse Large B Cell Non-Hodgkin's Lymphoma. U.S. National Institutes of Health. | |||||
| REF 25 | ClinicalTrials.gov (NCT05328102) A Phase 2 Randomized, Open-Label, Multicenter Study to Evaluate the Efficacy and Safety of XmAb13676 (Plamotamab) Combined With Tafasitamab Plus Lenalidomide Versus Tafasitamab Plus Lenalidomide in Subjects With Relapsed or Refractory Diffuse Large B-Cell Lymphoma. U.S.National Institutes of Health. | |||||
| REF 26 | ClinicalTrials.gov (NCT05685173) A Phase 1 Study to Assess Safety and Tolerability of REGN5837, an Anti-CD22 x Anti-CD28 Costimulatory Bispecific Monoclonal Antibody, in Combination With Odronextamab, an Anti-CD20 x Anti-CD3 Bispecific Monoclonal Antibody, in Patients With Aggressive B-Cell Non-Hodgkin Lymphomas (ATHENA-1). U.S.National Institutes of Health. | |||||
| REF 27 | ClinicalTrials.gov (NCT00354926) Safety and Efficacy Study of an Anti-CD20 Monoclonal Antibody (AME-133v) to Treat Non-Hodgkin's Lymphoma. U.S. National Institutes of Health. | |||||
| REF 28 | J Clin Oncol 30, 2012 (suppl, abstr 8081). | |||||
| REF 29 | Clinical pipeline report, company report or official report of MENTRIK Biotech. | |||||
| REF 30 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800037513) | |||||
| REF 31 | ClinicalTrials.gov (NCT03196830) CAR-T for R/R B-NHL | |||||
| REF 32 | ClinicalTrials.gov (NCT03888105) Assess the Anti-Tumor Activity and Safety of Odronextamab in Patients With Relapsed or Refractory B-cell Non-Hodgkin Lymphoma. U.S. National Institutes of Health. | |||||
| REF 33 | ClinicalTrials.gov (NCT00634933) Study Evaluating 2 Dosing Regimens Of TRU-015 In Rheumatoid Arthritis. U.S. National Institutes of Health. | |||||
| REF 34 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8268). | |||||
| REF 35 | ClinicalTrials.gov (NCT01390545) VELVET, a Dose Range Finding Trial of Veltuzumab in Subjects With Moderate to Severe Rheumatoid Arthritis. U.S. National Institutes of Health. | |||||
| REF 36 | ClinicalTrials.gov (NCT01735604) Genetically Engineered Lymphocyte Therapy in Treating Patients With Lymphoma That is Resistant or Refractory to Chemotherapy | |||||
| REF 37 | ClinicalTrials.gov (NCT04030195) Dose-escalation Study of Safety of PBCAR20A in Subjects With r/r NHL or r/r CLL/SLL. U.S. National Institutes of Health. | |||||
| REF 38 | ClinicalTrials.gov (NCT03625037) GEN3013 Trial in Patients With Relapsed, Progressive or Refractory B-Cell Lymphoma. U.S. National Institutes of Health. | |||||
| REF 39 | ClinicalTrials.gov (NCT03125577) Combination CAR-T Cell Therapy Targeting Hematological Malignancies | |||||
| REF 40 | ClinicalTrials.gov (NCT03207178) Sequential Infusion of Anti-CD19 and Anti-CD20 CAR-T Cells Against Relapsed and Refractory B-cell Lymphoma | |||||
| REF 41 | ClinicalTrials.gov (NCT02737085) the Sequential Therapy of CD19-targeted and CD20-targeted CAR-T Cell Therapy for Diffuse Large B Cell Lymphoma(DLBCL) | |||||
| REF 42 | ClinicalTrials.gov (NCT02710149) A Clinical Research of CD20-Targeted CAR-T in B Cell Malignancies | |||||
| REF 43 | ClinicalTrials.gov (NCT02965157) Pilot Study of Anti-CD20-CAR-engineered T Cells in Patients With Chemotherapy Resistant or Refractory CD20+ Lymphoma | |||||
| REF 44 | ClinicalTrials.gov (NCT03398967) A Feasibility and Safety Study of Universal Dual Specificity CD19 and CD20 or CD22 CAR-T Cell Immunotherapy for Relapsed or Refractory Leukemia and Lymphoma | |||||
| REF 45 | ClinicalTrials.gov (NCT03277729) A Phase I/II Study to Evaluate the Safety of Cellular Immunotherapy Using Autologous T Cells Engineered to Express a CD20-Specific Chimeric Antigen Receptor for Patients With Relapsed or Refractory B Cell Non-Hodgkin Lymphomas | |||||
| REF 46 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800030721) | |||||
| REF 47 | ClinicalTrials.gov (NCT03664635) MB-CART20.1 Lymphoma | |||||
| REF 48 | ClinicalTrials.gov (NCT04156178) CD20-CD19 Compound CAR (cCAR) T Cells for Patients With Relapsed /Refractory B Cell Malignancies. U.S. National Institutes of Health. | |||||
| REF 49 | ClinicalTrials.gov (NCT04082936) A Safety and Pharmacokinetic Study of IGM-2323 in Subjects With Relapsed/Refractory Non-Hodgkin Lymphoma. U.S. National Institutes of Health. | |||||
| REF 50 | ClinicalTrials.gov (NCT04989803) A Phase 1 Open-label, Multicenter Study Evaluating the Safety and Efficacy of KITE-363 or KITE-753, Autologous Anti-CD19/CD20 CAR T-cell Therapies, in Subjects With Relapsed and/or Refractory B-cell Lymphoma. U.S.National Institutes of Health. | |||||
| REF 51 | Clinical pipeline report, company report or official report of Roche | |||||
| REF 52 | ClinicalTrials.gov (NCT03271515) Immunotherapy With Bispecific CAR-T Cells for B-Cell Lymphoma, ALL and CLL | |||||
| REF 53 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800033790) | |||||
| REF 54 | ClinicalTrials.gov (NCT03375619) Long-term Follow-up Study of Patients Receiving CAR-20/19-T Cells | |||||
| REF 55 | ClinicalTrials.gov (NCT03291444) CAR-T Cells Combined With Peptide Specific Dendritic Cell in Relapsed/Refractory Leukemia/MDS | |||||
| REF 56 | ClinicalTrials.gov (NCT03576807) The Clinical Research of Anti-CD20 CAR-T Cells in Patients With Refractory or Relapsed B Lymphocyte Lymphoma | |||||
| REF 57 | ClinicalTrials.gov (NCT03407859) Sequential Treatment With CD20/CD22/CD10-CART After CD19-CART Treatment Base on MRD in Relapsed/Refractory B-ALL | |||||
| REF 58 | CD20-Targeted T Cells after Stem Cell Transplantation for High Risk and Refractory Non-Hodgkin's Lymphoma. Biol Blood Marrow Transplant. 2013 June; 19(6): 925-933. | |||||
| REF 59 | J Clin Oncol 27:15s, 2009 (suppl, abstr 8566). | |||||
| REF 60 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 61 | ClinicalTrials.gov (NCT03097770) Treatment of Relapsed and/or Chemotherapy Refractory B-cell Malignancy by Tandem CAR T Cells Targeting CD19 and CD20 | |||||
| REF 62 | Clinical pipeline report, company report or official report of Atara Biotherapeutics. | |||||
| REF 63 | Structure of the Fab fragment of therapeutic antibody Ofatumumab provides insights into the recognition mechanism with CD20. Mol Immunol. 2009 Jul;46(11-12):2419-23. | |||||
| REF 64 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2628). | |||||
| REF 65 | Knockouts model the 100 best-selling drugs--will they model the next 100 Nat Rev Drug Discov. 2003 Jan;2(1):38-51. | |||||
| REF 66 | The optimized anti-CD20 monoclonal antibody ublituximab bypasses natural killer phenotypic features in Waldenstr m macroglobulinemia. Haematologica. 2015 Apr;100(4):e147-51. | |||||
| REF 67 | Clinical pipeline report, company report or official report of Roche (2009). | |||||
| REF 68 | Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003 Oct 20;22(47):7359-68. | |||||
| REF 69 | Comparative nonclinical assessments of the proposed biosimilar PF-05280586 and rituximab (MabThera ). Toxicol Pathol. 2014 Oct;42(7):1069-81. | |||||
| REF 70 | Results of a phase 1 study of AME-133v (LY2469298), an Fc-engineered humanized monoclonal anti-CD20 antibody, in FcgammaRIIIa-genotyped patients with previously treated follicular lymphoma. Clin Cancer Res. 2012 Mar 1;18(5):1395-403. | |||||
| REF 71 | National Cancer Institute Drug Dictionary (drug id 599668). | |||||
| REF 72 | Rituximab blocks binding of radiolabeled anti-CD20 antibodies (Ab) but not radiolabeled anti-CD45 Ab. Blood. 2008 Aug 1;112(3):830-5. | |||||
| REF 73 | A phase I/II trial of iodine-131-tositumomab (anti-CD20), etoposide, cyclophosphamide, and autologous stem cell transplantation for relapsed B-cell lymphomas. Blood. 2000 Nov 1;96(9):2934-42. | |||||
| REF 74 | TRU-015, a small modular immunopharmaceutical (SMIP drug candidate directed against CD20, demonstrates clinical improvement in subjects with rheumatoid arthritis. Arthritis Res Ther. 2007; 9(Suppl 3): P32. | |||||
| REF 75 | Veltuzumab, an anti-CD20 mAb for the treatment of non-Hodgkin's lymphoma, chronic lymphocytic leukemia and immune thrombocytopenic purpura. Curr Opin Mol Ther. 2009 Apr;11(2):200-7. | |||||
| REF 76 | Immunotherapy with FBTA05 (Bi20), a trifunctional bispecific anti-CD3 x anti-CD20 antibody and donor lymphocyte infusion (DLI) in relapsed or refractory B-cell lymphoma after allogeneic stem cell transplantation: study protocol of an investigator-driven, open-label, non-randomized, uncontrolled, dose-escalating Phase I/II-trial. J Transl Med. 2013 Jul 2;11:160. | |||||
| REF 77 | BM-ca is a newly defined type I/II anti-CD20 monoclonal antibody with unique biological properties. Int J Oncol. 2011 Feb;38(2):335-44. | |||||
| REF 78 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||||
| REF 79 | Anti CD37 antibodies | |||||
| REF 80 | Structure of CD20 in complex with the therapeutic monoclonal antibody rituximab. Science. 2020 Mar 13;367(6483):1224-1230. | |||||
| REF 81 | Binding mechanisms of therapeutic antibodies to human CD20. Science. 2020 Aug 14;369(6505):793-799. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

