Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T79232
(Former ID: TTDS00109)
|
|||||
| Target Name |
Vasopressin V1a receptor (V1AR)
|
|||||
| Synonyms |
Vascular/hepatic-type arginine vasopressin receptor; V1aR; V1a vasopressin receptor; Antidiuretic hormone receptor 1a; AVPR1; AVPR V1a
Click to Show/Hide
|
|||||
| Gene Name |
AVPR1A
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 5 Target-related Diseases | + | ||||
| 1 | Acute diabete complication [ICD-11: 5A2Y] | |||||
| 2 | Autism spectrum disorder [ICD-11: 6A02] | |||||
| 3 | Hypertension [ICD-11: BA00-BA04] | |||||
| 4 | Hypo-osmolality/hyponatraemia [ICD-11: 5C72] | |||||
| 5 | Localisation [ICD-11: N.A.] | |||||
| Function |
The activity of this receptor is mediated by G proteins which activate a phosphatidyl-inositol-calcium second messenger system. Has been involved in social behaviors, including affiliation and attachment. Receptor for arginine vasopressin.
Click to Show/Hide
|
|||||
| BioChemical Class |
GPCR rhodopsin
|
|||||
| UniProt ID | ||||||
| Sequence |
MRLSAGPDAGPSGNSSPWWPLATGAGNTSREAEALGEGNGPPRDVRNEELAKLEIAVLAV
TFAVAVLGNSSVLLALHRTPRKTSRMHLFIRHLSLADLAVAFFQVLPQMCWDITYRFRGP DWLCRVVKHLQVFGMFASAYMLVVMTADRYIAVCHPLKTLQQPARRSRLMIAAAWVLSFV LSTPQYFVFSMIEVNNVTKARDCWATFIQPWGSRAYVTWMTGGIFVAPVVILGTCYGFIC YNIWCNVRGKTASRQSKGAEQAGVAFQKGFLLAPCVSSVKSISRAKIRTVKMTFVIVTAY IVCWAPFFIIQMWSVWDPMSVWTESENPTITITALLGSLNSCCNPWIYMFFSGHLLQDCV QSFPCCQNMKEKFNKEDTDSMSRRQTFYSNNRSPTNSTGMWKDSPKSSKSIKFIPVST Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A02189 ; BADD_A02787 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 4 Approved Drugs | + | ||||
| 1 | Felypressin | Drug Info | Approved | Localisation | [7] | |
| 2 | Mozavaptan | Drug Info | Approved | Hyponatraemia | [3], [8], [9] | |
| 3 | Oxytocin | Drug Info | Approved | Autism spectrum disorder | [10] | |
| 4 | Terlipressin | Drug Info | Approved | Hypertension | [11] | |
| Clinical Trial Drug(s) | [+] 7 Clinical Trial Drugs | + | ||||
| 1 | FE-202158 | Drug Info | Phase 2 | Sepsis | [14] | |
| 2 | PMX-53 | Drug Info | Phase 2 | Atopic dermatitis | [15], [16] | |
| 3 | RG7314 | Drug Info | Phase 2 | Autism spectrum disorder | [17] | |
| 4 | SRX-246 | Drug Info | Phase 2 | Anxiety disorder | [18] | |
| 5 | SSR149415 | Drug Info | Phase 2 | Anxiety disorder | [19], [20], [21] | |
| 6 | VA-111913 | Drug Info | Phase 2 | Dysmenorrhea | [22] | |
| 7 | SRX-251 | Drug Info | Phase 1 | Dysmenorrhea | [24] | |
| Discontinued Drug(s) | [+] 3 Discontinued Drugs | + | ||||
| 1 | OPC-21268 | Drug Info | Discontinued in Phase 2 | Cardiac disease | [25], [26] | |
| 2 | PF-3274167 | Drug Info | Discontinued in Phase 1 | Female sexual arousal dysfunction | [27] | |
| 3 | JTV-605 | Drug Info | Terminated | Dysmenorrhea | [29] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | NOX-F37 | Drug Info | Preclinical | Acute and chronic heart failure | [28] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| Agonist | [+] 7 Agonist drugs | + | ||||
| 1 | Felypressin | Drug Info | [30] | |||
| 2 | Oxytocin | Drug Info | [33] | |||
| 3 | FE-202158 | Drug Info | [35] | |||
| 4 | PMID25776143-Compound-1 | Drug Info | [42] | |||
| 5 | PMID25776143-Compound-2 | Drug Info | [42] | |||
| 6 | [Thr4,Gly7]OT | Drug Info | [33] | |||
| 7 | [Val4]AVP | Drug Info | [59] | |||
| Inhibitor | [+] 42 Inhibitor drugs | + | ||||
| 1 | Mozavaptan | Drug Info | [31], [32] | |||
| 2 | ATOSIBAN | Drug Info | [34] | |||
| 3 | PMX-53 | Drug Info | [36] | |||
| 4 | SSR149415 | Drug Info | [39] | |||
| 5 | PMID25776143-Compound-3 | Drug Info | [42] | |||
| 6 | PMID25776143-Compound-4 | Drug Info | [42] | |||
| 7 | Tetra-azabenzo[e]azulene derivative 1 | Drug Info | [42] | |||
| 8 | Tetra-azabenzo[e]azulene derivative 2 | Drug Info | [42] | |||
| 9 | PF-3274167 | Drug Info | [45] | |||
| 10 | L-371257 | Drug Info | [45] | |||
| 11 | A-987306 | Drug Info | [46] | |||
| 12 | ARGENINE VASOPRESSIN | Drug Info | [47] | |||
| 13 | D(CH2)5[Tyr(Me)2,Thr4,Orn8,Tyr9-NH2]VT | Drug Info | [48] | |||
| 14 | DesGly-NH2,d(CH2)5[D-Tyr2,Thr4,Orn8(5/6C-Flu)]VT | Drug Info | [48] | |||
| 15 | D[Arg4,Lys8]VP | Drug Info | [47] | |||
| 16 | D[Arg4,Orn8]VP | Drug Info | [47] | |||
| 17 | D[Arg4]AVP | Drug Info | [47] | |||
| 18 | D[Cha4,Dab8]VP | Drug Info | [47] | |||
| 19 | D[Cha4,Dap8]VP | Drug Info | [47] | |||
| 20 | D[Cha4,Lys8]VP | Drug Info | [47] | |||
| 21 | D[Cha4,Orn8]VP | Drug Info | [47] | |||
| 22 | D[Cha4]AVP | Drug Info | [47] | |||
| 23 | D[D-3-Pal2]AVP | Drug Info | [47] | |||
| 24 | D[Leu4,Dab8]VP | Drug Info | [47] | |||
| 25 | D[Leu4,Dap8]VP | Drug Info | [47] | |||
| 26 | D[Leu4,Lys8]VP | Drug Info | [47] | |||
| 27 | D[Leu4]AVP | Drug Info | [47] | |||
| 28 | D[Lys8(5/6-Flu)]VT | Drug Info | [48] | |||
| 29 | D[Orn4,Lys8]VP | Drug Info | [47] | |||
| 30 | D[Orn8(5/6C-Flu)]VT | Drug Info | [48] | |||
| 31 | D[Thr4,Lys8(5/6C-Flu)]VT | Drug Info | [48] | |||
| 32 | D[Thr4,Orn8(5/6C-Flu)]VT | Drug Info | [48] | |||
| 33 | HO-LVA | Drug Info | [51] | |||
| 34 | Hoo-Phe-Orn-Pro-hle-Pff-Phe-NH2 | Drug Info | [36] | |||
| 35 | L-372662 | Drug Info | [45] | |||
| 36 | Relcovaptan | Drug Info | [51], [54] | |||
| 37 | [HO1][Lys8(5/6C-Flu)]VT | Drug Info | [48] | |||
| 38 | [HO1][Orn8(5/6C-Flu)]VT | Drug Info | [48] | |||
| 39 | [HO1][Orn8(5/6C-Rhm)]VT | Drug Info | [48] | |||
| 40 | [HO1][Thr4,Orn8(5/6C-Flu)]VT | Drug Info | [48] | |||
| 41 | [Lys8(Alexa 488) ]PVA | Drug Info | [51] | |||
| 42 | [Lys8(Alexa 546) ]PVA | Drug Info | [51] | |||
| Stimulator | [+] 1 Stimulator drugs | + | ||||
| 1 | Terlipressin | Drug Info | [1] | |||
| Modulator | [+] 1 Modulator drugs | + | ||||
| 1 | RG7314 | Drug Info | [37] | |||
| Antagonist | [+] 19 Antagonist drugs | + | ||||
| 1 | SRX-246 | Drug Info | [38] | |||
| 2 | VA-111913 | Drug Info | [40] | |||
| 3 | SRX-251 | Drug Info | [41] | |||
| 4 | PMID25776143-Compound-7 | Drug Info | [42] | |||
| 5 | PMID25776143-Compound-8 | Drug Info | [42] | |||
| 6 | PMID28906174-Compound-figure1g | Drug Info | [43] | |||
| 7 | Pyrrolidine derivative 13 | Drug Info | [43] | |||
| 8 | OPC-21268 | Drug Info | [31], [44] | |||
| 9 | NOX-F37 | Drug Info | [28] | |||
| 10 | JTV-605 | Drug Info | [44] | |||
| 11 | CL-385004 | Drug Info | [44] | |||
| 12 | d(CH2)5[Tyr(Me)2]AVP | Drug Info | [49] | |||
| 13 | d[Pen1,Tyr(Me)2]AVP | Drug Info | [50] | |||
| 14 | L023103 | Drug Info | [52] | |||
| 15 | LS-192629 | Drug Info | [53] | |||
| 16 | SSR126768A | Drug Info | [55] | |||
| 17 | V1A F/U | Drug Info | [56] | |||
| 18 | YM 218 | Drug Info | [57] | |||
| 19 | YM 471 | Drug Info | [58] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Calcium signaling pathway | hsa04020 | Affiliated Target |
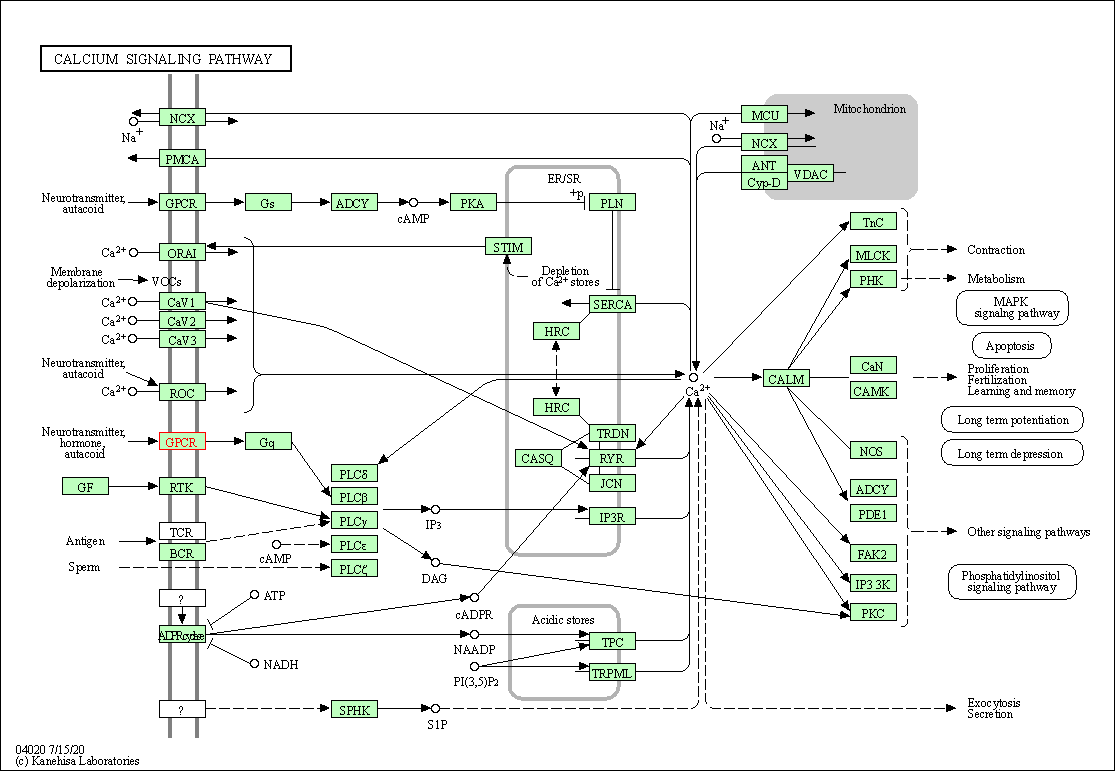
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Phospholipase D signaling pathway | hsa04072 | Affiliated Target |
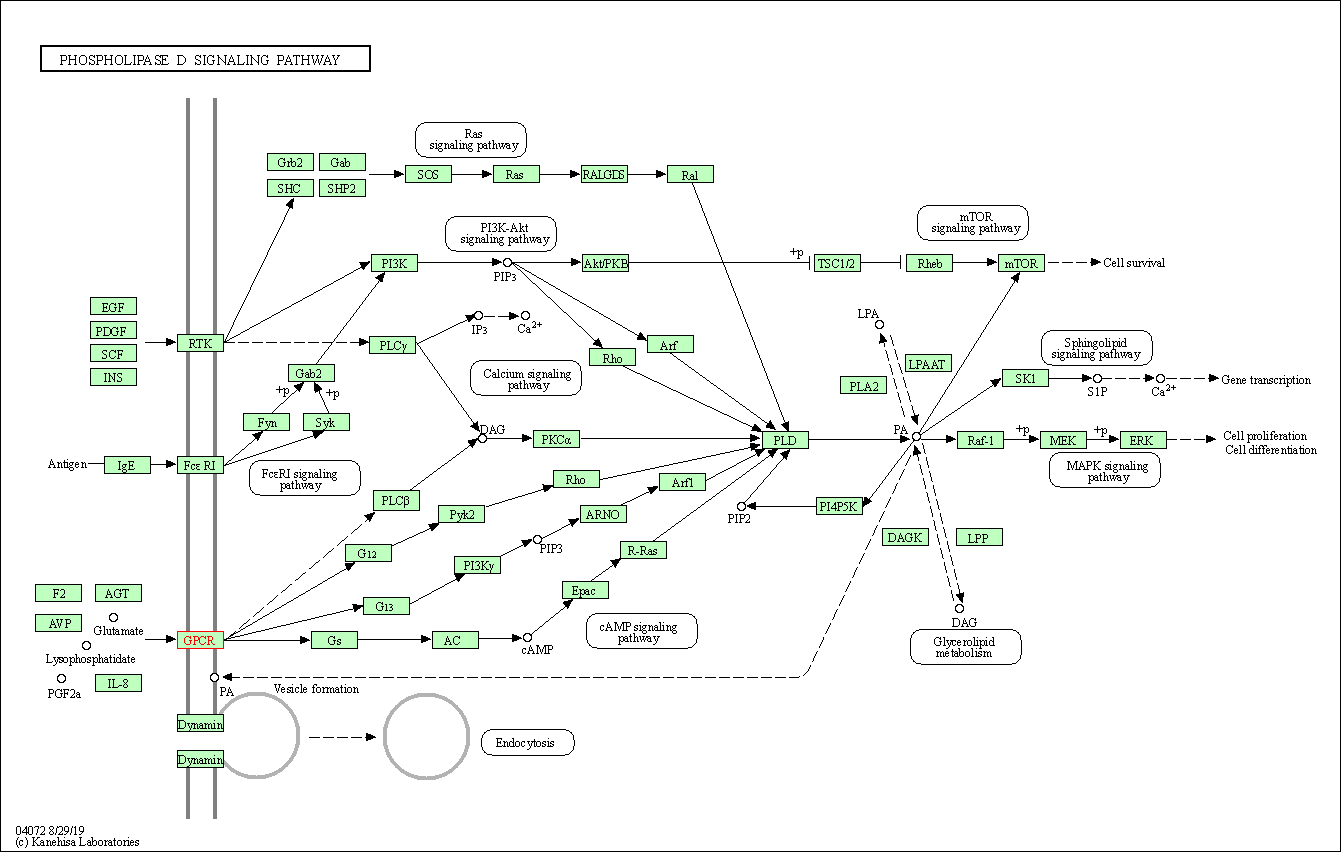
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Neuroactive ligand-receptor interaction | hsa04080 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Vascular smooth muscle contraction | hsa04270 | Affiliated Target |
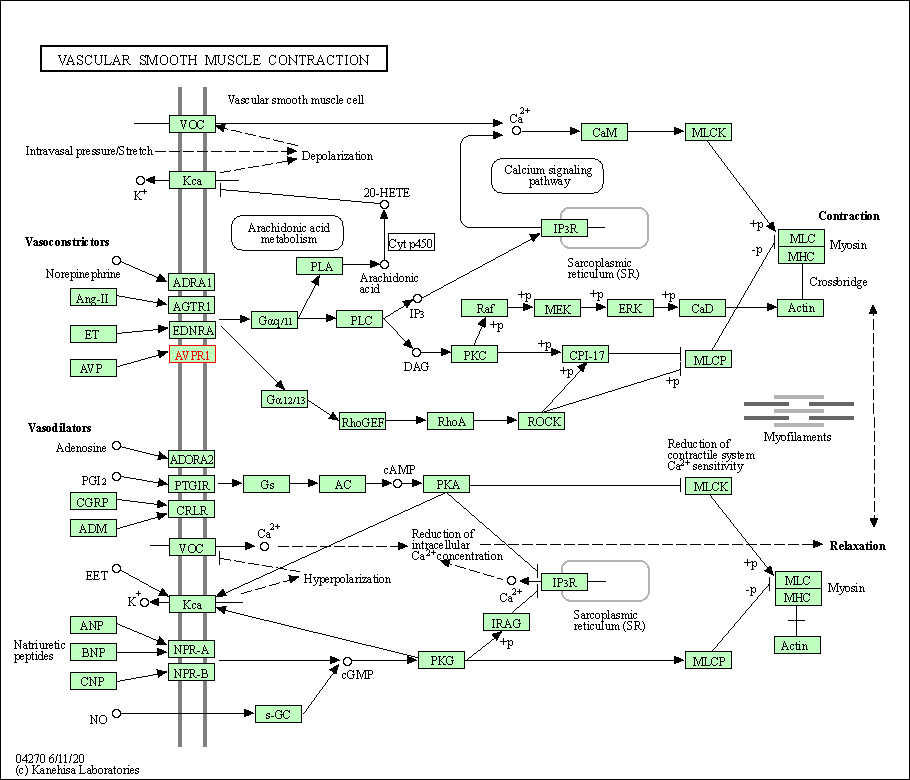
|
| Class: Organismal Systems => Circulatory system | Pathway Hierarchy | ||
| Degree | 2 | Degree centrality | 2.15E-04 | Betweenness centrality | 2.73E-07 |
|---|---|---|---|---|---|
| Closeness centrality | 1.45E-01 | Radiality | 1.18E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 2.00E+00 | Topological coefficient | 5.00E-01 | Eccentricity | 14 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 3 KEGG Pathways | + | ||||
| 1 | Calcium signaling pathway | |||||
| 2 | Neuroactive ligand-receptor interaction | |||||
| 3 | Vascular smooth muscle contraction | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Vasopressin-like receptors | |||||
| 2 | G alpha (q) signalling events | |||||
| WikiPathways | [+] 5 WikiPathways | + | ||||
| 1 | GPCRs, Class A Rhodopsin-like | |||||
| 2 | Gastrin-CREB signalling pathway via PKC and MAPK | |||||
| 3 | Peptide GPCRs | |||||
| 4 | GPCR ligand binding | |||||
| 5 | GPCR downstream signaling | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Investigational vasopressin receptor modulators in the pipeline. Expert Opin Investig Drugs. 2009 Aug;18(8):1119-31. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2182). | |||||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 4 | ClinicalTrials.gov (NCT00748072) 1-deamino 8-d-arginine Vasopressin (DDAVP) in Percutaneous Ultrasound-guided Renal Biopsy. U.S. National Institutes of Health. | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2203). | |||||
| REF 6 | Emerging diuretics for the treatment of heart failure. Expert Opin Emerg Drugs. 2009 Mar;14(1):195-204. | |||||
| REF 7 | Drug Therapy in Nursing. 2008. By Diane S. Aschenbrenner, Samantha J. Venable. | |||||
| REF 8 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001739) | |||||
| REF 9 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2197). | |||||
| REF 10 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2174). | |||||
| REF 11 | Pharmacology, clinical efficacy and safety of terlipressin in esophageal varices bleeding, septic shock and hepatorenal syndrome. Expert Rev Gastroenterol Hepatol. 2007 Dec;1(2):207-17. | |||||
| REF 12 | ClinicalTrials.gov (NCT03504917) A Study of Balovaptan in Adults With Autism Spectrum Disorder With a 2-Year Open-Label Extension. U.S. National Institutes of Health. | |||||
| REF 13 | ClinicalTrials.gov (NCT02507284) Tolerability, Safety, and Activity of SRX246 in Irritable Subjects With Huntington's Disease. U.S. National Institutes of Health. | |||||
| REF 14 | ClinicalTrials.gov (NCT01612676) Investigating FE 202158 as Potential Primary Treatment in Patients With Early Septic Shock. U.S. National Institutes of Health. | |||||
| REF 15 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 579). | |||||
| REF 16 | PMX-53 as a dual CD88 antagonist and an agonist for Mas-related gene 2 (MrgX2) in human mast cells. Mol Pharmacol. 2011 Jun;79(6):1005-13. | |||||
| REF 17 | ClinicalTrials.gov (NCT01793441) A Study of RG7314 to Investigate Efficacy and Safety in Individuals With Autism Spectrum Disorders. U.S. National Institutes of Health. | |||||
| REF 18 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 19 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2202). | |||||
| REF 20 | Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for t... Proc Natl Acad Sci U S A. 2002 Apr 30;99(9):6370-5. | |||||
| REF 21 | Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharmacology. 2008 Aug;33(9):2080-92. | |||||
| REF 22 | ClinicalTrials.gov (NCT00963053) VA111913 Dysmenorrhoea Efficacy and Safety Proof of Concept. U.S. National Institutes of Health. | |||||
| REF 23 | Clinical pipeline report, company report or official report of Neumora Therapeutics | |||||
| REF 24 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025117) | |||||
| REF 25 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2196). | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000284) | |||||
| REF 27 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025596) | |||||
| REF 28 | Emerging drugs for acute and chronic heart failure: current and future developments. Expert Opin Emerg Drugs. 2007 Mar;12(1):75-95. | |||||
| REF 29 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010443) | |||||
| REF 30 | Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473-512. | |||||
| REF 31 | Nonpeptide vasopressin antagonists: a new group of hormone blockers entering the scene. Exp Clin Endocrinol Diabetes. 1999;107(3):157-65. | |||||
| REF 32 | New analgesic drugs derived from phencyclidine. J Med Chem. 1981 May;24(5):496-9. | |||||
| REF 33 | Mapping peptide-binding domains of the human V1a vasopressin receptor with a photoactivatable linear peptide antagonist. J Biol Chem. 1997 Oct 17;272(42):26536-44. | |||||
| REF 34 | The discovery of GSK221149A: a potent and selective oxytocin antagonist. Bioorg Med Chem Lett. 2008 Jan 1;18(1):90-4. | |||||
| REF 35 | The selective vasopressin type 1a receptor agonist selepressin (FE 202158) blocks vascular leak in ovine severe sepsis*. Crit Care Med. 2014 Jul;42(7):e525-33. | |||||
| REF 36 | Peptidomimetic C5a receptor antagonists with hydrophobic substitutions at the C-terminus: increased receptor specificity and in vivo activity. Bioorg Med Chem Lett. 2006 Oct 1;16(19):5088-92. | |||||
| REF 37 | Social Communication is an Emerging Target for Pharmacotherapy in Autism Spectrum Disorder - A Review of the Literature on Potential Agents. J Can Acad Child Adolesc Psychiatry. 2014 February; 23(1):20-30. | |||||
| REF 38 | Pharmacokinetics and metabolism of SRX246: a potent and selective vasopressin 1a antagonist. J Pharm Sci. 2013 Jun;102(6):2033-43. | |||||
| REF 39 | Tetrahydroquinoline sulfonamides as vasopressin 1b receptor antagonists. Bioorg Med Chem Lett. 2009 Nov 1;19(21):6018-22. | |||||
| REF 40 | Clinical pipeline report, company report or official report of Avarx. | |||||
| REF 41 | Orally active vasopressin V1a receptor antagonist, SRX251, selectively blocks aggressive behavior. Pharmacol Biochem Behav. 2006 Feb;83(2):169-74. | |||||
| REF 42 | Vasopressin V1a and V1b receptor modulators: a patent review (2012 - 2014).Expert Opin Ther Pat. 2015 Jun;25(6):711-22. | |||||
| REF 43 | A patent review of oxytocin receptor antagonists 2013-2017.Expert Opin Ther Pat. 2017 Dec;27(12):1287-1290. | |||||
| REF 44 | Nonpeptide vasopressin receptor antagonists: development of selective and orally active V1a, V2 and V1b receptor ligands. Prog Brain Res. 2002;139:197-210. | |||||
| REF 45 | Oral oxytocin antagonists. J Med Chem. 2010 Sep 23;53(18):6525-38. | |||||
| REF 46 | cis-4-(Piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quinazolin-2-amine (A-987306), a new histamine H4R antagonist that blocks pain... J Med Chem. 2008 Nov 27;51(22):7094-8. | |||||
| REF 47 | Design and synthesis of the first selective agonists for the rat vasopressin V(1b) receptor: based on modifications of deamino-[Cys1]arginine vasop... J Med Chem. 2007 Feb 22;50(4):835-47. | |||||
| REF 48 | Synthesis and characterization of fluorescent antagonists and agonists for human oxytocin and vasopressin V(1)(a) receptors. J Med Chem. 2002 Jun 6;45(12):2579-88. | |||||
| REF 49 | Conserved aromatic residues in the transmembrane region VI of the V1a vasopressin receptor differentiate agonist vs. antagonist ligand binding. Eur J Biochem. 2000 Jul;267(13):4253-63. | |||||
| REF 50 | Pharmacological characterization of the human vasopressin receptor subtypes stably expressed in Chinese hamster ovary cells. Br J Pharmacol. 1998 Dec;125(7):1463-70. | |||||
| REF 51 | Toward efficient drug screening by homogeneous assays based on the development of new fluorescent vasopressin and oxytocin receptor ligands. J Med Chem. 2007 Oct 4;50(20):4976-85. | |||||
| REF 52 | Discovery and development of a new class of potent, selective, orally active oxytocin receptor antagonists. J Med Chem. 2005 Dec 1;48(24):7882-905. | |||||
| REF 53 | Pharmacology of (2S,4Z)-N-[(2S)-2-hydroxy-2-phenylethyl]-4-(methoxyimino) -1-[(2'-methyl[1,1'-biphenyl]-4-yl)carbonyl]-2-pyrrolidinecarboxamide, a ... J Pharmacol Exp Ther. 2003 Jul;306(1):253-61. | |||||
| REF 54 | Binding of [3H] SR 49059, a potent nonpeptide vasopressin V1a antagonist, to rat and human liver membranes. Biochem Biophys Res Commun. 1994 Feb 28;199(1):353-60. | |||||
| REF 55 | SSR126768A (4-chloro-3-[(3R)-(+)-5-chloro-1-(2,4-dimethoxybenzyl)-3-methyl-2-oxo-2,3-dihydro-1H-indol-3-yl]-N-ethyl-N-(3-pyridylmethyl)-benzamide, ... J Pharmacol Exp Ther. 2004 Apr;309(1):414-24. | |||||
| REF 56 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 366). | |||||
| REF 57 | Effects of YM218, a nonpeptide vasopressin V1A receptor-selective antagonist, on human vasopressin and oxytocin receptors. Pharmacol Res. 2005 Mar;51(3):275-81. | |||||
| REF 58 | Effects of YM471, a nonpeptide AVP V(1A) and V(2) receptor antagonist, on human AVP receptor subtypes expressed in CHO cells and oxytocin receptors in human uterine smooth muscle cells. Br J Pharmacol. 2001 Jul;133(5):746-54. | |||||
| REF 59 | [1-deamino-4-cyclohexylalanine] arginine vasopressin: a potent and specific agonist for vasopressin V1b receptors. Endocrinology. 2002 Dec;143(12):4655-64. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

