Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T97766
(Former ID: TTDI02423)
|
|||||
| Target Name |
Leukocyte surface antigen CD47 (CD47)
|
|||||
| Synonyms |
Protein MER6; MER6; Integrinassociated protein; Integrin-associated protein; IAP; Antigenic surface determinant protein OA3
Click to Show/Hide
|
|||||
| Gene Name |
CD47
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Myelodysplastic syndrome [ICD-11: 2A37] | |||||
| 2 | Stomach cancer ICD-11: 2B72 | |||||
| Function |
Plays an important role in memory formation and synaptic plasticity in the hippocampus. Receptor for SIRPA, binding to which prevents maturation of immature dendritic cells and inhibits cytokine production by mature dendritic cells. Interaction with SIRPG mediates cell-cell adhesion, enhances superantigen-dependent T-cell-mediated proliferation and costimulates T-cell activation. May play a role in membrane transport and/or integrin dependent signal transduction. May prevent premature elimination of red blood cells. May be involved in membrane permeability changes induced following virus infection. Has a role in both cell adhesion by acting as an adhesion receptor for THBS1 on platelets, and in the modulation of integrins.
Click to Show/Hide
|
|||||
| BioChemical Class |
Osteoclast fusion complex
|
|||||
| UniProt ID | ||||||
| Sequence |
MWPLVAALLLGSACCGSAQLLFNKTKSVEFTFCNDTVVIPCFVTNMEAQNTTEVYVKWKF
KGRDIYTFDGALNKSTVPTDFSSAKIEVSQLLKGDASLKMDKSDAVSHTGNYTCEVTELT REGETIIELKYRVVSWFSPNENILIVIFPIFAILLFWGQFGIKTLKYRSGGMDEKTIALL VAGLVITVIVIVGAILFVPGEYSLKNATGLGLIVTSTGILILLHYYVFSTAIGLTSFVIA ILVIQVIAYILAVVGLSLCIAACIPMHGPLLISGLSILALAQLLGLVYMKFVASNQKTIQ PPRKAVEEPLNAFKESKGMMNDE Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 3 Clinical Trial Drugs | + | ||||
| 1 | Hu5F9-G4 | Drug Info | Phase 3 | Myelodysplastic syndrome | [2] | |
| 2 | ALX148 | Drug Info | Phase 1/2 | Myelodysplastic syndrome | [7] | |
| 3 | TTI-621 | Drug Info | Phase 1 | Melanoma | [1] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Inhibitor | [+] 3 Inhibitor drugs | + | ||||
| 1 | Hu5F9-G4 | Drug Info | [13], [14] | |||
| 2 | ALX148 | Drug Info | [14] | |||
| 3 | TTI-621 | Drug Info | [1] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| ECM-receptor interaction | hsa04512 | Affiliated Target |
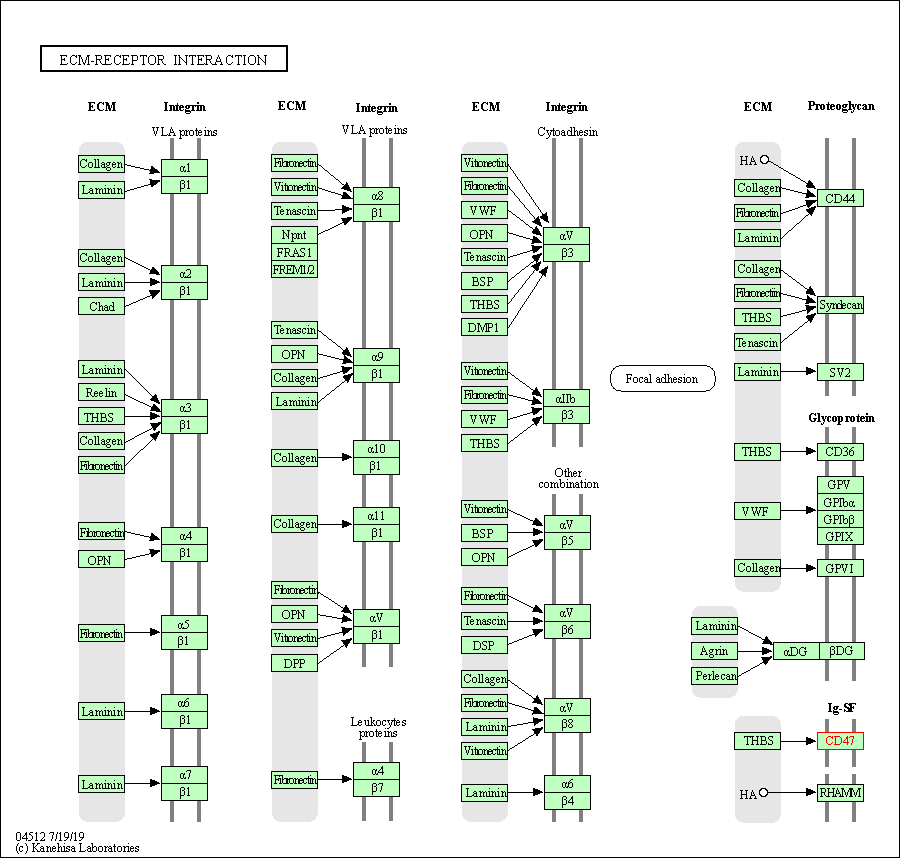
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Degree | 9 | Degree centrality | 9.67E-04 | Betweenness centrality | 3.15E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.27E-01 | Radiality | 1.40E+01 | Clustering coefficient | 1.94E-01 |
| Neighborhood connectivity | 3.52E+01 | Topological coefficient | 1.45E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 2 | ClinicalTrials.gov (NCT04313881) Magrolimab + Azacitidine Versus Azacitidine + Placebo in Untreated Participants With Myelodysplastic Syndrome (MDS) (ENHANCE). U.S. National Institutes of Health. | |||||
| REF 3 | ClinicalTrials.gov (NCT05002127) A Phase 2/3 Study of Evorpacept (ALX148) in Patients With Advanced HER2-Overexpressing Gastric/Gastroesophageal Junction Adenocarcinoma (ASPEN-06). U.S.National Institutes of Health. | |||||
| REF 4 | ClinicalTrials.gov (NCT05626322) A PHASE 1b/2 STUDY OF PF-07901801, A CD47 BLOCKING AGENT, WITH TAFASITAMAB AND LENALIDOMIDE FOR PARTICIPANTS WITH RELAPSED/REFRACTORY DIFFUSE LARGE B CELL LYMPHOMA NOT ELIGIBLE FOR STEM CELL TRANSPLANTATION. U.S.National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT04440735) A Study of DSP107 Alone and in Combination With Atezolizumab for Patients With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT03834948) AO-176 in Multiple Solid Tumor Malignancies. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT04417517) A Study of ALX148 With Azacitidine for Higher Risk Myelodysplastic Syndrome (ASPEN-02). U.S. National Institutes of Health. | |||||
| REF 8 | Clinical pipeline report, company report or official report of I-MAB Biopharma. | |||||
| REF 9 | ClinicalTrials.gov (NCT04306224) A Study of IMC-002 in Subjects With Metastatic or Locally Advanced Solid Tumors and Relapsed or Refractory Lymphomas. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT03530683) A Trial of TTI-622 in Patients With Advanced Relapsed or Refractory Lymphoma or Myeloma (TTI-622-01). U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT04257617) A Trial of ZL-1201 in Subjects With Advanced Cancer. U.S. National Institutes of Health. | |||||
| REF 12 | ClinicalTrials.gov (NCT04511975) A Study Evaluating the Safety and Efficacy of IBI188 in Combination With AZA in Subjects With Newly Diagnosed MDS. U.S. National Institutes of Health. | |||||
| REF 13 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 14 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 15 | ClinicalTrials.gov (NCT03804996) Study of TG-1801 in Subjects With B-Cell Lymphoma. U.S. National Institutes of Health. | |||||
| REF 16 | ClinicalTrials.gov (NCT04406623) Phase 1 Study of SL-172154 (SIRPalpha-Fc-CD40L) in Subjects With Ovarian Cancer. U.S. National Institutes of Health. | |||||
| REF 17 | ClinicalTrials.gov (NCT04881045) A PHASE 1 DOSE ESCALATION AND EXPANSION STUDY EVALUATING THE SAFETY, TOLERABILITY, PHARMACOKINETICS, PHARMACODYNAMICS, AND ANTITUMOR ACTIVITY OF PF-07257876 IN PATIENTS WITH ADVANCED OR METASTATIC TUMORS. U.S.National Institutes of Health. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

