Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01MML
|
||||
| Former ID |
DNCL002314
|
||||
| Drug Name |
MLN9708
|
||||
| Synonyms |
Ixazomib
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
Millennium Pharmaceuticals
|
||||
| Structure |
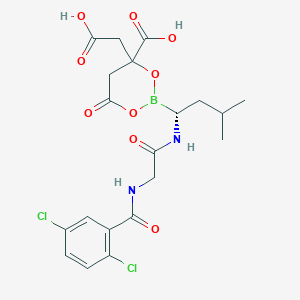
|
Download2D MOL |
|||
| Formula |
C14H19BCl2N2O4
|
||||
| InChI |
InChI=1S/C14H19BCl2N2O4/c1-8(2)5-12(15(22)23)19-13(20)7-18-14(21)10-6-9(16)3-4-11(10)17/h3-4,6,8,12,22-23H,5,7H2,1-2H3,(H,18,21)(H,19,20)/t12-/m0/s1
|
||||
| InChIKey |
MXAYKZJJDUDWDS-LBPRGKRZSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
57291607, 85029113, 104253504, 124757626, 124955652, 125164430, 135268985, 135626848, 136340279, 136348738, 136367593, 138100925, 144116241, 152134539, 162011779, 162037798, 162202726, 162773434, 163326535, 163347633, 163908014, 164193936, 164834143, 174007116, 174526063, 177748936, 184814067, 198939582, 203355961, 210274689, 210280323, 223389604, 229732516, 248181513, 249810660, 252110098, 252166660, 252213184, 252443130
|
||||
| Target and Pathway | |||||
| Target(s) | Proteasome | Target Info | Modulator | [530730] | |
| References | |||||
| Ref 524820 | ClinicalTrials.gov (NCT02181413) A Study of Oral Ixazomib Citrate (MLN9708) Maintenance Therapy in Patients With Multiple Myeloma Following Autologous Stem Cell Transplant. U.S. National Institutes of Health. | ||||
| Ref 543142 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8450). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

