Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04ADG
|
||||
| Former ID |
DCL001004
|
||||
| Drug Name |
SU5416
|
||||
| Synonyms |
Semaxanib; Semaxinib; Semaxnib; Semoxind; S 8442; SU 5416; VEGF Receptor 2 Kinase Inhibitor III; InSolution™ SU-5416; TSU-16; Semaxanib (USAN/INN); Methylene]-2H-indol-2-one; (3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-1,3-dihydro-2H-indol-2-one; (3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-1H-indol-2-one; 1,3-Dihydro-3-[(3,5-dimethyl-1H-pyrrol-2-yl); 1,3-Dihydro-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylene]-2H-indol-2-one; 3-((Z)-(3,5-Dimethylpyrrol-2-yl)methylene)-2-indolinone; 3-(1-(3,5-Dimethyl-1H-pyrrol-2-yl)meth-(Z)-ylidene)-2-oxo-2,3-dihydroindole; 3-(2,4-dimethylpyrrol-5-yl)methylidene-indolin-2-one; 3-[(2,4-Dimethylpyrrol-5-yl)methylidene]-indolin-2-one; 3-[(2,4-dimethylpyrrol-5-yl)methylidenyl]indolin-2-one; 3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-1,3-dihydro-2H-indol-2-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
Sugen
|
||||
| Structure |
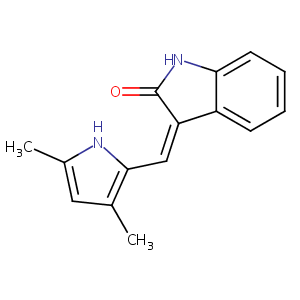
|
Download2D MOL |
|||
| Formula |
C15H14N2O
|
||||
| InChI |
InChI=1S/C15H14N2O/c1-9-7-10(2)16-14(9)8-12-11-5-3-4-6-13(11)17-15(12)18/h3-8,16H,1-2H3,(H,17,18)/b12-8-
|
||||
| InChIKey |
WUWDLXZGHZSWQZ-WQLSENKSSA-N
|
||||
| CAS Number |
CAS 204005-46-9
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
525180, 8034458, 10318718, 11061325, 12015099, 14847387, 17405666, 24278606, 26759729, 26759733, 39301723, 47207480, 47348964, 47572946, 49674962, 49984916, 50076383, 50107014, 50107015, 50107016, 53778216, 53789746, 56312333, 56312335, 56312337, 56312516, 56312518, 56312520, 56320689, 56322916, 57361292, 74446362, 85789116, 90341564, 91722386, 92303403, 99302833, 103175684, 113911626, 121362026, 121493282, 124750244, 124800944, 124891688, 134339165, 134339435, 134340578, 134341737, 135079569, 135141433
|
||||
| Target and Pathway | |||||
| Target(s) | Aryl hydrocarbon receptor | Target Info | Modulator | [527804] | |
| References | |||||
| Ref 468143 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5056). | ||||
| Ref 527804 | A Phase I study of the angiogenesis inhibitor SU5416 (semaxanib) in solid tumours, incorporating dynamic contrast MR pharmacodynamic end points. Br J Cancer. 2005 Oct 17;93(8):876-83. | ||||
| Ref 537114 | Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

