Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D06WPM
|
||||
| Former ID |
DIB004329
|
||||
| Drug Name |
Coprexa
|
||||
| Synonyms |
Tetrathiomolybdate
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Neurological disease [ICD9: 338, 338.2, 410, 782.3,780; ICD10:I21, I22, R52, R52.1-R52.2, R60.9, G89] | Phase 3 | [528378] | ||
| Company |
Pipex Pharmaceuticals
|
||||
| Structure |
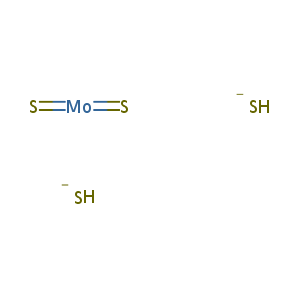
|
Download2D MOL |
|||
| Formula |
H2MoS4-2
|
||||
| Canonical SMILES |
[SH-].[SH-].S=[Mo]=S
|
||||
| InChI |
1S/Mo.2H2S.2S/h;2*1H2;;/p-2
|
||||
| InChIKey |
VVRHUOPINLMZBL-UHFFFAOYSA-L
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Superoxide dismutase [Cu-Zn] | Target Info | Inhibitor | [528378] | |
| BioCyc Pathway | Reactive oxygen species degradation | ||||
| NetPath Pathway | TCR Signaling Pathway | ||||
| Pathway Interaction Database | Validated nuclear estrogen receptor alpha network | ||||
| FOXA1 transcription factor network | |||||
| PathWhiz Pathway | Degradation of Superoxides | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

