Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D07IQS
|
||||
| Former ID |
DAP000706
|
||||
| Drug Name |
Atazanavir
|
||||
| Synonyms |
ATV; ATZ; Atazanavirum; Latazanavir; Reyataz; Zrivada; BMS 232632; CGP 73547; DR7; Atazanavir (INN); Atazanavir [INN:BAN];BMS-232632; CGP-73547; Reyataz (TN); Reyataz, BMS-232632, Atazanavir; Reyataz(TM) (*1:1 sulfate*); Dimethyl (3S,8S,9S,12S)-9-benzyl-3,12-di-tert-butyl-8-hydroxy-4,11-dioxo-6-[4-(2-pyridyl)benzyl]-2,5,6,10,13-pentaazatetradecanedioate; METHYL [(1S,4S,5S,10S)-4-BENZYL-1,10-DI-TERT-BUTYL-5-HYDROXY-2,9,12-TRIOXO-7-(4-PYRIDIN-2-YLBENZYL)-13-OXA-3,7,8,11-TETRAAZATETRADEC-1-YL]CARBAMATE; Methyl N-[(2S)-1-[2-[(2S,3S)-2-hydroxy-3-[[(2S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoyl]amino]-4-phenylbutyl]-2-[(4-pyridin-2-ylphenyl)methyl]hydrazinyl]-3,3-dimethyl-1-oxobutan-2-yl]carbamate; (2S)-N-(3-{[(2S)-2-(Methoxycarbonylamino)-3,3-dimethylbutanoylamino][(4-(2-pyridyl)phenyl)methyl]amino}(1S,2S)-2-hydroxy-1-benzylpropyl)-2-(methoxycarbonylamino)-3,3-dimethylbutanamide; (3S,8S,9S,12S)-3,12-BIS(1,1-DIMETHYLETHYL)-8-HYDROXY-4,11-DIOXO-9-(PHENYLMETHYL)-6-[[4-(2-PYRIDINYL)PHENYL]METHYL]-2,5,6,10,13-PENTAAZATETRADECANEDIOIC ACID DIMETHYL ESTER
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Human immunodeficiency virus infection [ICD9: 279.3; ICD10:B20-B26] | Approved | [536361] | ||
| Therapeutic Class |
Anti-HIV Agents
|
||||
| Company |
Bristol-Myers Squibb
|
||||
| Structure |
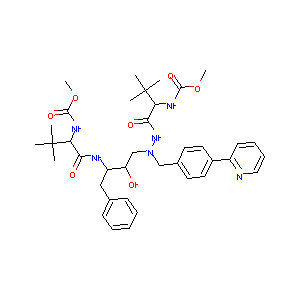
|
Download2D MOL |
|||
| Formula |
C38H52N6O7
|
||||
| Canonical SMILES |
CC(C)(C)C(C(=O)NC(CC1=CC=CC=C1)C(CN(CC2=CC=C(C=C2)C3=CC<br />=CC=N3)NC(=O)C(C(C)(C)C)NC(=O)OC)O)NC(=O)OC
|
||||
| InChI |
1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1
|
||||
| InChIKey |
AXRYRYVKAWYZBR-GASGPIRDSA-N
|
||||
| CAS Number |
CAS 198904-31-3
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
621982, 10249838, 14715932, 14766361, 14790731, 15047322, 17436380, 24276961, 46225140, 46393831, 46508504, 46512956, 49831615, 50067727, 50070909, 50780515, 51091799, 53801241, 57346722, 85177037, 85177040, 85177051, 85177056, 87225781, 92308998, 92729699, 99437162, 103338831, 104418454, 124757264, 124894049, 125164068, 125267482, 126649083, 126665840, 126731250, 127338999, 127339000, 127474087, 134337995, 134339780, 135105507, 135692446, 137004575, 142410398, 143496681, 144206632, 152234924, 152344127, 160647610
|
||||
| ChEBI ID |
ChEBI:37924
|
||||
| SuperDrug ATC ID |
J05AE08
|
||||
| Target and Pathway | |||||
| Target(s) | HIV protease | Target Info | Modulator | [556264] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

