Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D09ZXO
|
||||
| Former ID |
DNC000432
|
||||
| Drug Name |
Cilengitide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
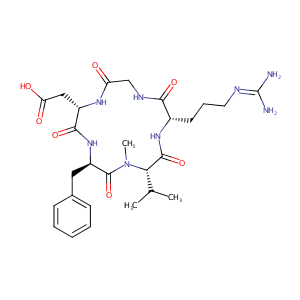
|
Download2D MOL |
|||
| Formula |
C27H40N8O7
|
||||
| InChI |
InChI=1S/C27H40N8O7/c1-15(2)22-25(41)33-17(10-7-11-30-27(28)29)23(39)31-14-20(36)32-18(13-21(37)38)24(40)34-19(26(42)35(22)3)12-16-8-5-4-6-9-16/h4-6,8-9,15,17-19,22H,7,10-14H2,1-3H3,(H,31,39)(H,32,36)(H,33,41)(H,34,40)(H,37,38)(H4,28,29,30)/t17-,18-,19+,22-/m0/s1
|
||||
| InChIKey |
AMLYAMJWYAIXIA-VWNVYAMZSA-N
|
||||
| CAS Number |
CAS 188968-51-6
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
825381, 12015138, 14764661, 14886950, 17397633, 33500423, 49956880, 50950930, 53790369, 57288492, 57288711, 57395325, 103570373, 104056223, 104425945, 126648372, 126666747, 134339858, 135156087, 137156914, 137262614, 162220925, 162808281, 163621005, 163686336, 164178308, 178103210, 179150114, 184823749, 198962979, 219812641, 223662751, 226088004, 226420604, 243159770, 249582596, 249583895, 252215145, 252471106
|
||||
| Target and Pathway | |||||
| Target(s) | Integrin alpha-V/beta-3 | Target Info | Modulator | ||
| References | |||||
| Ref 522337 | ClinicalTrials.gov (NCT00689221) Cilengitide, Temozolomide, and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma and Methylated Gene Promoter Status. U.S. National Institutes of Health. | ||||
| Ref 541715 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6597). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

