Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0D7QO
|
||||
| Former ID |
DIB013991
|
||||
| Drug Name |
Phenylephrine hydrochloride gel
|
||||
| Synonyms |
Incostop; Phenylephrine hydrochloride; Phenylephrine hydrochloride gel (fecal incontinence); VEN-308; Phenylephrine hydrochloride gel (fecal incontinence), SLA Pharma/Ventrus Biosciences; 10% phenylephrine HCl cream, Solvay/SLA Pharma; 20% phenylephrine HCl cream, Solvay/SLA Pharma
|
||||
| Indication | Fecal incontinence [ICD10:R15] | Approved | [547809] | ||
| Company |
SLA Pharma UK Ltd
|
||||
| Structure |
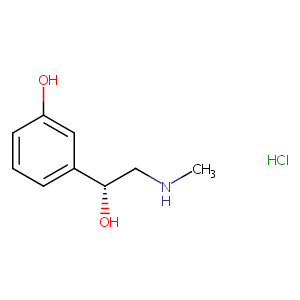
|
Download2D MOL |
|||
| Canonical SMILES |
c1(cc(O)ccc1)[C@@H](O)CNC.Cl
|
||||
| CAS Number |
CAS 61-76-7
|
||||
| Target and Pathway | |||||
| Target(s) | Alpha-1D adrenergic receptor | Target Info | Modulator | [527269] | |
| NetPath Pathway | IL2 Signaling Pathway | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

