Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0J8JP
|
||||
| Former ID |
DCL000930
|
||||
| Drug Name |
Pimavanserin
|
||||
| Synonyms |
ST51054136; AC-5273; I14-1981; 1-[(4-fluorophenyl)methyl]-1-(1-methyl-4-piperidyl)-3-[[4-(2-methylpropoxy)phenyl]methyl]urea
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
Acadia Pharmaceuticals
|
||||
| Structure |
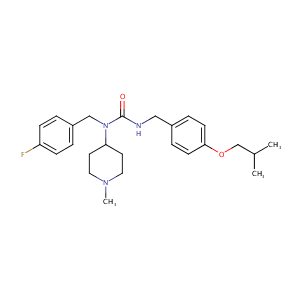
|
Download2D MOL |
|||
| Formula |
C25H34FN3O2
|
||||
| InChI |
InChI=1S/C25H34FN3O2/c1-19(2)18-31-24-10-6-20(7-11-24)16-27-25(30)29(23-12-14-28(3)15-13-23)17-21-4-8-22(26)9-5-21/h4-11,19,23H,12-18H2,1-3H3,(H,27,30)
|
||||
| InChIKey |
RKEWSXXUOLRFBX-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 706779-91-1
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
15056570, 22487415, 44188211, 78486719, 85246183, 92721414, 118844935, 125356530, 126619173, 126661719, 131324331, 134222314, 134339374, 135262066, 135263922, 137005043, 137354663, 152042012, 162104969, 162205223, 163242432, 163884831, 172914648, 174539041, 187072850, 198956315, 210275101, 210280740, 223435110, 223679488, 224220742, 226970761, 241139662, 242550037, 248856106, 249870692, 252166633, 252216077, 252448725
|
||||
| Target and Pathway | |||||
| Target(s) | 5-hydroxytryptamine 2A receptor | Target Info | Modulator | ||
| Atypical antipsychotic | Target Info | Modulator | [889440] | ||
| PANTHER Pathway | 5HT2 type receptor mediated signaling pathway | ||||
| References | |||||
| Ref 543125 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8423). | ||||
| Ref 547301 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800014997) | ||||
| Ref 889440 | 2016 FDA drug approvals. Nat Rev Drug Discov. 2017 Feb 2;16(2):73-76. doi: 10.1038/nrd.2017.14. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

